Scroll to:
Regulation of the oil receptivity of the surface of diamonds and kimberlite minerals using various classes of regulating agents
https://doi.org/10.17073/2500-0632-2025-09-454
Abstract
The purpose of the research is to select appropriate agents for regulating the oil receptivity of diamond and kimberlite mineral surfaces in the conditioning of diamond-kimberlite products prior to their beneficiation by froth flotation and X-ray luminescence separation using phosphor-containing modifying agents and collecting agents, the basis of which is apolar collecting agents. The paper presents the results of comprehensive physicochemical studies of the influence of various classes of regulating agents on the attachment of apolar collecting agents on the surface of diamonds and kimberlite minerals (visiometric analysis, measurement of wetting contact angles in a mineral–organic collecting agent–aqueous phase system, measurement of surface tension at the organic collecting agent–aqueous phase–frothless flotation phase boundary). Based on the analysis of the data obtained, effective regulating agents have been identified and selected to ensure the selectivity of diamond beneficiation. Regulating agents belonging to the classes of alkylarylphosphonates (NTPA, OEDPA), aminopolycarboxylic acids (EDTA), cationic polymers (PEG-1500, Neonol AF-9-6), polyphosphates (STPP), bifunctionally modified carboxymethylcellulose derivatives (CMC 75-V and Kamcel-600), mixtures of alkyl phosphates, alkyl phosphonates, and anionic polymers (IS-3), ionogenic and non-ionogenic nitrogen-containing polymers (Emulsifier OP-4, Oxypav A1218.30), amino alcohols (TEA), hydroxy acids (lactic acid), and quaternary ammonium bases (ammonium sulfate) were tested. Talc, pyrite, calcite, muscovite, phlogopite, serpentine, and dolomite were selected as the main minerals of kimberlite prone to adhesion of apolar collecting agents. It has been established that the wetting contact angle is reduced most significantly by the agents Neonol AF-9-6, Emulsifier OP-4, and Oxypav A1218.30 that is associated with a significant decrease in the surface tension of the interface between an organic collecting agent and the aqueous phase. A thermodynamic assessment of the oil receptivity of kimberlite minerals conducted using the Dupré–Young equation and based on measurements of wetting contact angles and surface tension showed that the energy of adhesion of an organic collecting agent on kimberlite minerals with the addition of regulating agents decreases by 2–6 times and reaches values of 6–17 J/m2, approaching the adhesion energy of water (5 J/m2). On diamonds, the energy of adhesion of an apolar collecting agent at maximum concentrations decreases only to 17–27.5 J/m2 that determines its stable attachment. The results of flotation tests confirmed the depressing ability of the studied regulating agents in relation to the flotation-responsing minerals of kimberlite. Based on the analysis of the data obtained, effective regulating agents have been selected and recommended for testing in industrial froth separation modes, ensuring increased selectivity of apolar collecting agent attachment on the surface of diamonds and kimberlite minerals: NTPA, OEDPA, IS-3, OP-4.
Keywords
For citations:
Chanturiya V.A., Morozov V.V., Chanturiya E.L., Samusev A.L. Regulation of the oil receptivity of the surface of diamonds and kimberlite minerals using various classes of regulating agents. Mining Science and Technology (Russia). 2025;10(4):379–392. https://doi.org/10.17073/2500-0632-2025-09-454
Regulation of the oil receptivity of the surface of diamonds and kimberlite minerals using various classes of regulating agents
Introduction
The current task in developing a process for extracting weakly and abnormally luminescent diamonds in X-ray luminescence separation processes is to increase the selectivity of attachment of phosphor-containing modifying agents on the surface of diamonds and the rational selection of regulating agents preventing the attachment of apolar collecting agents, which are part of the modifying agents used, on the hydrophobic minerals of kimberlite [1, 2]. A similar objective, increasing the selectivity of attachment of apolar collecting agents on the surface of diamonds, is a prerequisite for improving the efficiency of flotation and froth separation processes [3, 4]. In both cases, in order to select effective agent modes, it is necessary to establish the regularities of attachment of organic collecting agents and regulating agents that regulate hydrophobicity and oil receptivity on diamonds and kimberlite minerals [1, 5].
The purpose of the research is to select appropriate agents for regulating the oil receptivity of diamond and kimberlite mineral surfaces in the conditioning of diamond-kimberlite products prior to their beneficiation by froth flotation and X-ray luminescence separation using phosphor-containing modifying agents and collecting agents, the basis of which is apolar collecting agents.
The objectives of the research were:
- identification of kimberlite minerals most prone to adhesion of organic collecting agent drops;
- determining the influence of regulating agents on the adhesion energy of apolar collecting agents on the surface of diamonds and oleophilic kimberlite minerals;
- selection of regulating agents that maximally increase the contrast in the flotation response of diamonds and kimberlite minerals.
The basis for selecting research methods and approaches to solving the task at hand is an analysis of current scientific and technical developments and research in the field of regulating surface processes in water-mineral disperse systems [6, 7].
A significant number of studies in this area have been conducted in relation to the processes of froth flotation and separation of diamonds from kimberlites1 [8, 9]. Along with the selection of collecting agents, the presented works recommended a specific set of regulating agents designed to suppress the floatability of kimberlite.
The results of research in related fields were also used to make an informed choice of regulating agents. The studies related to the use of layered aluminosilicate minerals as sorbents for heavy metals and petroleum products from wastewater [10] are similar in nature to the task at hand. However, due to the inverse final objective (increasing the sorption of pollutants), most of the theoretical and experimental provisions of these works only partially describe hydrophilization processes.
Research into the methods of increasing oil recovery from oil reservoirs is also of interest, particularly with regard to the use of regulating agents that help reduce the hydrophobicity of reservoir minerals, including layered aluminosilicate minerals [11]. However, due to the different mineral composition of the host rocks, the characteristics of the organic phase (oil), temperature, and pressure, most of the theoretical principles and experimental data are only of limited applicability for describing the hydrophilization processes of kimberlite minerals.
Another area in which a similar task of reducing the fouling of working surfaces with hardness salts is being addressed is the process of agent conditioning of aqueous media in thermal units and reverse osmosis plants [12].
Despite the differences between the processes considered and those occurring during processing of diamond-containing products prior to X-ray luminescence and froth separation processes, the similarity of the tasks to be solved – increasing the hydrophilicity of rock minerals and cleaning the surface of working elements from hydrophilic coatings – is the basis for selecting the range of regulating agents for the research being conducted.
To make an informed choice of agents that regulate the selectivity of attachment of agents modifying X-ray spectral characteristics and collecting agents on the surface of diamonds in the processes of X-ray luminescence and froth separation, a complex of physical and chemical research methods and thermodynamic modeling of the action of regulating agents on the attachment of organic collecting agents on hydrophobic kimberlite minerals in high-salt waters was used.
1 Yun R.-Kh., Kuznetsov D. Method for Extracting Diamonds from Vein Minerals. RF Patent No. 2412901, C2, publ. on 27.02.2011, Bul. No. 6 (In Russ.)
Research Techniques
The following objects were selected for the research: diamond crystals with polished surfaces, kimberlites of different degrees of metamorphism, as well as polished sections and plates of the most significant kimberlite minerals. CCHGO (cat cracking heavy gas oil) with additives of bunker fuel (BF) and luminol was used as a collecting agent. The studies were conducted using solutions of twelve regulating agents of different classes in model high-salt water, similar in composition to reclaim waters of beneficiation plants processing diamond-bearing kimberlites.
To preliminarily assess the intensity of organic collecting agent attachment on kimberlite minerals, a vision-based method [2] was used, which included treating a sample with an emulsion of a phosphor-containing organic collecting agent and obtaining and analyzing images of the mineral surface under ultraviolet light. The intensity of an organic collecting agent attachment to the surface of diamonds, individual minerals, and kimberlite grains was determined by analyzing images of the surface area occupied by the organic collecting agent after treatment of mineral samples. To measure the proportion of the surface occupied by the collecting agent (the degree of coverage), the areas with characteristic luminescence (ν = 500 nm) corresponding to the radiation of a mixture of CCHGO with BF and luminol were recorded.
To evaluate the influence of regulating agents on the oil receptivity of kimberlite minerals, wetting contact angles were measured in the mineral–organic collecting agent–aqueous phase system [13]. Methods for measuring wetting contact angle have previously been used to evaluate the restoration of hydrophobicity in natural diamonds through various types of physical and physicochemical treatment [14]. A DSA25 instrument equipped with image processing software2 was used to measure the wetting contact angle.
The built-in program determined the wetting contact angle at the moment of stabilization of the mineral – organic collecting agent drop – aqueous phase system (Fig. 1, a).
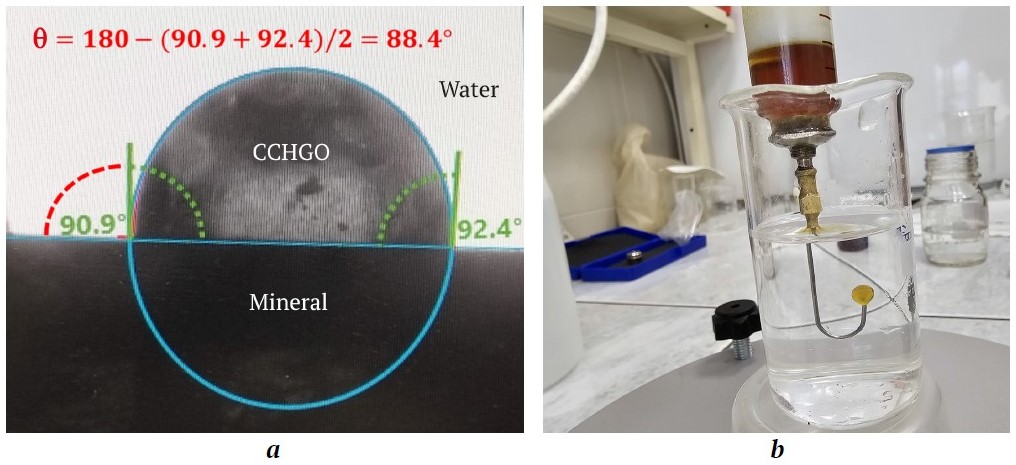
Fig. 1. Image of a drop of organic collecting agent, CCHGO (cat cracking heavy gas oil): a – on the surface of a mineral polished section with the results of measuring the wetting contact angle on the DSA25 instrument; b – on the curved capillary of the ST-1 stalagmometer
After each measurement, the surface of a diamond was cleaned using toluene and a 1N hydrochloric acid solution. To measure the wetting contact angle on kimberlite minerals, polished sections or plates of a mineral obtained by splitting natural samples were used.
The wetting contact angle in the apolar liquid–mineral–aqueous phase system is an informative quantitative characteristic of oil receptivity and is widely used to describe the wetting process of various surfaces in technological processes [15]. To study the attachment of a collecting agent on minerals in an aqueous phase, a dynamic method for measuring three-phase wetting contact angle was used [4, 16]. According to the method used, the surface of a mineral sample was first held in an aqueous phase of a specified composition, then the level of the aqueous phase in the cuvette was lowered to the level of the sample surface, and a drop of an organic collecting agent of a specified volume was applied. After applying a drop of a collecting agent to the surface of a sample in the cuvette, the liquid level was increased. At the same time, part of the drop detached from the surface of the sample and settled at the interface between the aqueous phase and the air. The wetting contact angle on the three-phase wetting perimeter was measured after equilibrium was established without removing the aqueous phase. The method used simulates the process of attachment of an apolar collecting agent on a mineral in a turbulent environment typical for the conditioning of diamond-kimberlite products prior to X-ray luminescence and froth separation processes.
To calculate the adhesion energy of an apolar collecting agent on the surface of minerals using the Dupré–Young equation, the surface tension of the interface between the organic collecting agent and the aqueous phase was determined. The measurements were performed using a ST-1 stalagmometer by determining the volume of floating drops of the collecting agent that detached from the curved capillary (Fig. 1, b), in accordance with the standard method3. The surface tension at the interface between the aqueous phase and air was measured by determining the volume of falling drops from a straight capillary in accordance with the method.
The flotation of diamonds, minerals, and kimberlite grains was carried out using the frothless flotation method in a Hallimond tube. The experimental method included preparing a mineral sample, preparing a liquid phase (high-salt water), water conditioning with a regulating agent, mineral sample conditioning with a collecting agent in water containing an oil receptivity regulating agent, flotation of diamonds, kimberlite, or kimberlite minerals, dewatering, drying, and weighing of the flotation products [4].
2 Drop Shape Analyzer DSA25 Specifications. URL: https://www.kruss-scientific.com/files/kruss-techdata-dsa25-en.pdf/
3 GOST R 50097–92 "Surfactants. Determination of Interfacial Tension. Drop Volume Method"
Visiometric analysis of organic collecting agent attachment on samples of kimberlite mineral components
At the initial stage of the research, kimberlite minerals were selected that are characterized by significant adhesion of the apolar collecting agent (CCHGO with BF and luminol) from aqueous emulsions. Minerals most commonly found in kimberlites of different degree of metamorphism were selected for the research. Model high-salt water was used to obtain the collecting agent emulsion. The concentration of the phosphor-containing collecting agent in the emulsion was 200 mg/L. The treatment time was 1 min. After the treatment and removing excess emulsion, the mineral sample was rinsed with reclaim water for 30 seconds.
The analysis of the images of mineral samples under ultraviolet light revealed significant differences in the intensity of organic collecting agent attachment on their surfaces: from almost complete coverage (talc, see Fig. 1) to complete absence of attachment (chromite, Fig. 2).
The results of the visiometric measurements allowed to select seven kimberlite minerals with high and medium degrees of coverage by the apolar collecting agent for further research (Table 1).
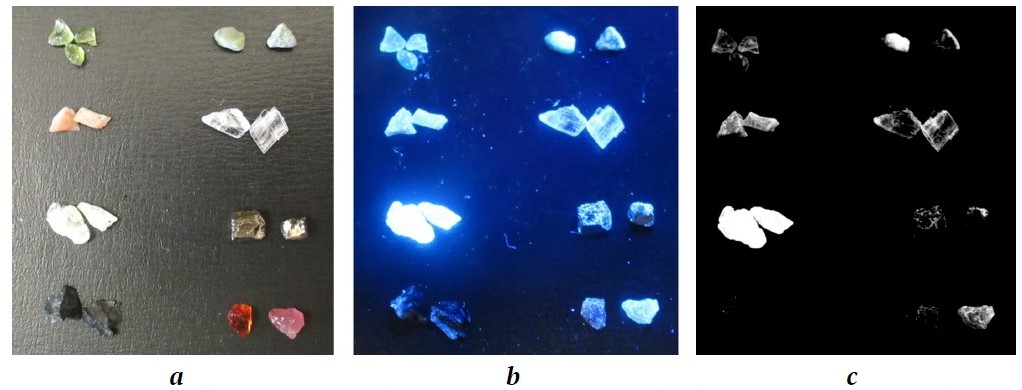
Fig. 2. Images and results of visiometric analysis of the attachment of an organic collecting agent with dissolved phosphor on the surface of kimberlite minerals and associated minerals:
a – in daylight; b – in UV light after treatment with a collecting agent; c – distribution of an organic collecting agent across mineral samples. Here (from right to left, top to bottom): olivine, calcite, celestine, muscovite, talc, pyrite, chromite, pyrope
Table 1
Results of visiometric analysis of apolar collecting agent attachment on kimberlite minerals
No. | Mineral | Prevalence in kimberlite, % | Percentage of surface area covered by organic collecting agent, % |
1 | Diamond | 2–10 carats/t | 56–95 |
Kimberlite minerals | |||
2 | Calcite | 1–40 | 22–65 |
3 | Muscovite | 0.1–5 | 25–50 |
4 | Talc | 0.1–5 | 80–93 |
5 | Pyrite | 0.05–1 | 35–55 |
6 | Phlogopite | 0.1–5 | 15–25 |
7 | Serpentine | 1–10 | 7–10 |
8 | Dolomite | 0.5–15 | 7–12 |
Minerals associated with diamonds that have a relatively high tendency to adhesion with organic collecting agents (celestine, pyrope, etc.) were not studied, as their contents in kimberlite are very insignificant.
Selection of agents for regulating the oil receptivity of kimberlite minerals
When selecting the initial range of agents for regulating the oil receptivity of kimberlite minerals, both traditional and new classes of organic compounds used to regulate the properties of minerals during flotation were considered: phosphorus-containing chelates, bifunctional cationic and anionic polymers, hydroxy acids, amino alcohols, bifunctionally modified carboxymethylcellulose derivatives, cationic nitrogen-containing olefins, aliphatic amines, quaternary ammonium compounds, and cationic polymers4 [8]. Initially, the list for the research included the agents used in related industries. For instance, the agents of the alkylarylphosphonate, nitrogen-containing olefin, and aliphatic amine classes are effectively used in oil production to increase oil recovery by reducing the hydrophobicity of reservoir minerals [17, 18]. The selection criteria were the requirements of commercial production of the agents of the listed classes and validation in flotation processes and related industries. In accordance with these requirements, 14 agents presented in Table 2 were selected for the studies.
A preliminary assessment of the effectiveness of the regulating agents under consideration was carried out by determining the "critical" concentration at which the attachment of an organic collecting agent ceases. The criterion for the effectiveness of regulating agents was their ability to minimize the wetting contact angle to the point of removing an organic collecting agent drop from the surface of a mineral sample when treated with regulating agent solutions in the concentration range from 10 to 1000 mg/L. Phlogopite was selected as an indicator mineral.
The results of the studies showed that a significant decrease in the wetting contact angle and detachment of an organic collecting agent drop from the surface of phlogopite in the specified concentration range is achieved when using 13 regulating agents (with the exception of ammonium sulfate). Therefore, all these agents were selected for subsequent tests (Table 2).
Table 2
Critical concentrations of regulating agents for attachment of organic collecting agent on phlogopite
No. | Regulating agent (of technical grade) | Class of organic compounds | Critical concentration of regulating agent, mg/L | |
with partial detachment of a collecting agent drop | with complete detachment of a collecting agent drop | |||
1 | Zinc complexonate of nitrilotrimethylphosphonic acid (NTPA) | Nitrilalkyl triphosphonate | 130 | 166 |
2 | Oxyethylidenediphosphonic acid (OEDPA) | Alkyl diphosphonate | 90 | 130 |
3 | Ethylendiaminetetraacetic acid (EDTA) | Aminopolycarbonic acid | 130 | 166 |
4 | Polyethylene glycol (PEG-1500) | Cationic polymer | 90 | 130 |
5 | Sodium tripolyphosphate (STPP) | Polyphosphate | 90 | 130 |
6 | Polyethylene glycol ether of monoalkyl phenols (Neonol AF-9-6) | Cationic nitrogen-containing polymer | 50 | 70 |
7 | Modified carboxymethylcellulose (CMC 75-V) | Bifunctionally modified CMC derivatives | 70 | 90 |
8 | Compound action antiscalant (AKVA-IS3) | A mixture of alkyl phosphates, alkyl phosphonates, and anionic polymers | 130 | 170 |
9 | Technical glyoxylic sodium carboxymethylcellulose (Kamcel-600) | Bifunctionally modified CMC derivatives | 50 | 70 |
10 | Alkyl C8-12-phenol ethoxylated (Emulsifier OP-4) | Short-chain aliphatic amine | 50 | 70 |
11 | Alkyldimethylamine oxideC12–C18 (Oxipav A1218.30) | Nonionic nitrogen-containing polymer | 70 | 90 |
12 | Triethanolamine (TEA) | Amino alcohol | 500 | 1,000 |
13 | 2-hydroxypropanoic acid (Lactic acid) | Hydroxy acid | 330 | 500 |
14 | Ammonium sulfate | Quaternary ammonium compound | – | – |
Research and thermodynamic analysis of the influence of regulating agents on the oil receptivity of kimberlite minerals
The thermodynamically justified parameter of the wetting ability of an organic collecting agent and, at the same time, the oil receptivity of a mineral surface is the work (energy) of adhesion. The work of adhesion of an apolar collecting agent on a mineral surface can be calculated using the Dupré–Young equation [19]:
Wоc–m = σоc–w(1 – cos θ), (1)
where Woc–m is the work of adhesion of a collecting agent on a mineral, J/m2; σoc–w is the interfacial tension at the interface between an organic collecting agent and an aqueous phase, N/m; θ is the three-phase wetting contact angle for a drop of organic collecting agent on a mineral surface in an aqueous phase, degrees.
To calculate the adhesion of an apolar collecting agent on a mineral surface, the wetting contact angles on diamond and kimberlite minerals (see Table 1) were measured using a DSA25 instrument in the aqueous phase (model high-salt water) with regulating agents dissolved in it in the concentration range of 0–500 mg/L. A mixture of CCHGO and BF (85 and 15%) was used as an organic collecting agent. During preliminary wetting of the polished sections, high-salt model water with additives of regulating agents was also used. A drop of an organic collecting agent was applied to the surface of a moistened mineral sample using a DSA25 dispenser syringe, after which the aqueous phase level in the cuvette was raised and images of a collecting agent drop attached to the surface of the mineral sample were obtained (see Fig. 1, a).
Next, using a ST-1 stalagmometer according to the method described in GOST R 50097–925, the mass and volume of ten drops of an organic collecting agent, which detached themselves from a curved capillary in an aqueous medium were measured. Based on the data obtained, the cell constant K (measured using kerosene) was found and the surface tension at the interface between an organic collecting agent and the aqueous phase (σoc–w) was determined for different agents in the concentration range of 0–500 mg/L:
K = 47.5/(V(ρwater − ρkerosene)); (2)
σoc–w = KV(ρw – ρoc), (3)
where V is the volume of a droplet, m3; ρwater is density of water, kg/m3; ρkerosene is density of kerosene, kg/m3; 47.5 is the surface tension of kerosene-water interface, mN/m; ρw is density of aqueous phase, kg/m3; ρoc is density of organic collecting agent, kg/m3.
The results of measuring the wetting contact angle showed that the selected agents affect the wettability of the surface of diamond, talc, calcite, and muscovite (Fig. 3, a, b) with an apolar collecting agent to different extent. It has been established that the agent Neonol AF-9-6 reduces the wetting contact angle of kimberlite minerals to the greatest extent. However, when using this agent, the wetting contact angle decreases on the surface of diamonds too. An effect on a diamond that differs from other agents is observed when emulsifier OP-4 is added: after an initial decrease in the wetting contact angle in the concentration range of 0–130 mg/L, a noticeable increase in the measured value is observed at higher concentrations (see Fig. 3, a).
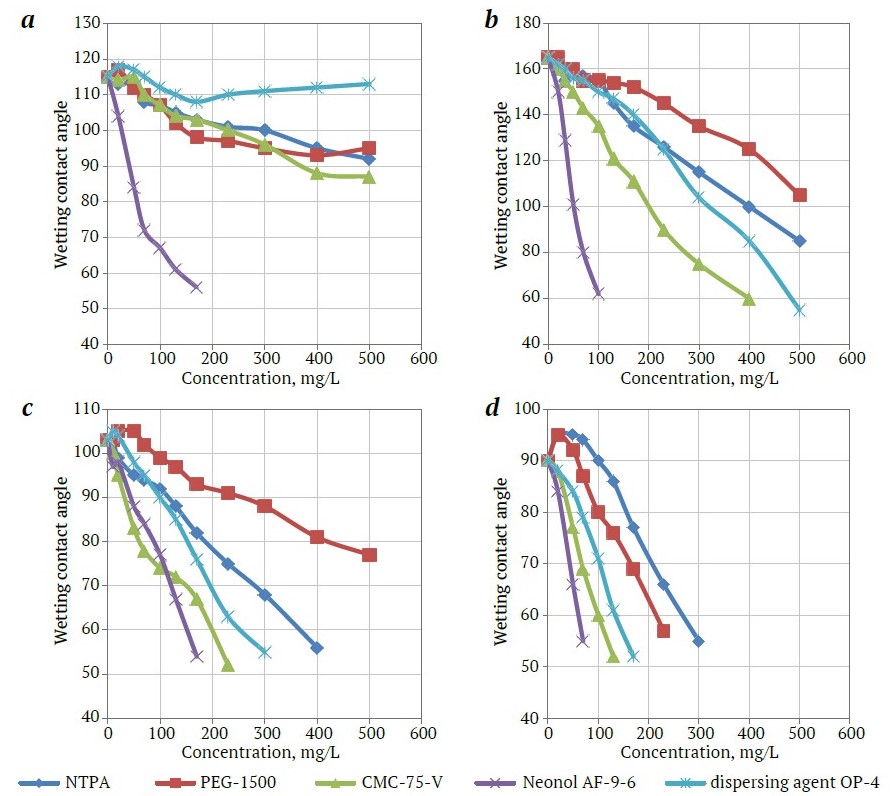
Fig. 3. The effect of regulating agents on wetting contact angle for the three-phase wetting perimeter of mineral – organic collecting agent – aqueous phase for diamond (a), talc (b), calcite (c), and muscovite (d): 1 – NTPA; 2 – PEG-1500; 3 – CMC-75-V; 4 – Neonol AF-9-6; 5 – dispersing agent OP-4
The minimal decrease in the wetting contact angle on kimberlite minerals is observed with the addition of alkylarylphosphonates (using NTPA as an example) and aliphatic alcohols (using PEG-1500 as an example). However, it should be noted that the decrease in the wetting contact angle when using these agents is significantly greater than on diamonds that indicates the potential benefit from their application. Organic polymers based on carboxymethylcellulose have the property of effectively reducing the oil receptivity of kimberlite minerals, but they also have a similar effect (to a lesser extent) on the attachment of apolar collecting agents on diamonds (see Fig. 3, curves 3), which allows them to be used at very low concentrations.
The results of surface tension measurements at the collecting agent–aqueous phase interface, partially presented in Table 3, indicate differences in the action of the studied regulating agents. The agents representing the classes of ionogenic and non-ionogenic nitrogen-containing polymers (Neonol AF 9-6 and Oxypav A1218.30) and short-chain aliphatic amine (OP-4) exhibit surfactant properties, reducing the surface tension at the interface between an organic collecting agent and aqueous phase by 2.8–3 times (see Table 3).
Table 3
Results of measuring and calculating surface tension at the interface between organic collecting agent and aqueous phase (model high-salt water)
Regulating agents | Concentration, mg/l | ||||||
0 | 50 | 100 | 170 | 230 | 300 | 500 | |
Surface tension, mN/m | |||||||
NTPA | 30.43 | 30.08 | 28.04 | 27.44 | 27.04 | 26.56 | 26.08 |
OEDPA | 30.43 | 27.43 | 25.34 | 22.99 | 21.65 | 20.34 | 20.17 |
EDTA | 30.43 | 30.13 | 30.01 | 29.60 | 29.14 | 26.33 | 22.08 |
PEG-1500 | 30.43 | 28.45 | 27.05 | 24.83 | 24.00 | 24.45 | 24.60 |
STPP | 30.43 | 26.20 | 25.24 | 25.01 | 24.78 | 25.12 | 22.50 |
Neonol AF 9-6 | 30.43 | 24.21 | 20.14 | 16.32 | 15.91 | 14.23 | 12.33 |
CMC 75-V | 30.43 | 28.34 | 27.77 | 27.22 | 26.95 | 25.45 | 23.47 |
IS-3 | 30.43 | 30.01 | 29.07 | 28.56 | 28.34 | 27.77 | 27.40 |
KAMCEL-600 | 30.43 | 28.56 | 28.13 | 27.89 | 27.73 | 27.10 | 27.30 |
OP-4 | 30.43 | 23.11 | 19.10 | 16.24 | 15.22 | 13.56 | 11.65 |
OXYPAV A1218.30 | 30.43 | 20.66 | 14.34 | 11.01 | 9.52 | 9.45 | 9.04 |
Triethanolamine | 30.43 | 28.33 | 27.40 | 26.22 | 26.00 | 26.04 | 25.91 |
Lactic acid | 30.43 | 28.00 | 27.25 | 27.02 | 26.86 | 25.34 | 23.30 |
Agents of other classes exhibit significantly lower surfactant properties, reducing surface tension by10–25% at a concentration of 300–500mg/L. The results of the surface tension measurements at the interface between an organic collecting agent and the aqueous phase in the presence of regulating agents were used to calculate the adhesion energy using equation (1).
The results of the calculations of adhesion energy on diamond, talc, calcite, and muscovite presented in Fig. 4 show that the agents studied reduce the adhesion energy of the apolar collecting agent on kimberlite minerals to different extent.

Fig. 4. The effect of regulating agents on the energy (work) of adhesion of an apolar collecting agent on diamond (a), talc (b), calcite (c), and muscovite (d): 1 – NTPA; 2 – PEG-1500; 3 – CMC-75-V; 4 – Neonol AF-9-6; 5 – OP-4 dispersing agent
The adhesion energy of the organic collecting agent on diamond with the addition of regulating agents (except for Neonol AF-9-6) decreases by 1.5–2 times to values of 17–27.5 J/m² (see Fig. 4, a). On kimberlite minerals, the adhesion energy of the organic collecting agent decreases by 2.5–6 times and reaches values of 6–17 J/m2 (see Fig. 4, b, c, d). Neonol AF-9-6 has the unusual ability to sharply reduce the adhesion energy of organic collecting agents on all samples, including diamonds.
To justify the hydrophilization of minerals under the influence of regulating agents, it seems appropriate to compare the adhesion energy of the organic collecting agent at "pre-critical" (before a collecting agent drop detaches) concentrations of regulating agents and the adhesion energy of the aqueous phase. The measurement results showed that for muscovite, the measured value of the wetting contact angle of a model water drop in air was 38 degrees and, accordingly, the calculated adhesion energy of the aqueous phase was 5.7 J/m2. The value obtained is close to the adhesion energy at "pre-critical" concentrations of regulating agents, ranging from 7 to 10.5 J/m² (see Fig. 4, d). Extrapolating the dependence of the adhesion energy on concentration to the "critical" concentration gives an adhesion energy value at the moment of a drop detachment of 5–7.5 J/m2. This result allows to conclude that the detachment of a drop of the organic collecting agent from the mineral surface, i.e., its hydrophilization, occurs when the adhesion energies of water and the apolar collecting agent on the mineral are equal or close in value.
4 Yun R.-Kh., Kuznetsov D. Method for Extracting Diamonds from Vein Minerals. RF Patent No. 2412901, C2, published on 27.02.2011, Bul. No. 6 (in Russian).
5 GOST R 50097–92 (ISO 9101–87) "Surfactants. Determination of Interfacial Tension. Drop Volume Method"
Studies of the influence of regulating agents on the floatability of kimberlite minerals using an apolar collecting agent
The general regularities described above regarding the influence of regulating agents on the attachment of an organic collecting agent have been confirmed by the results of the studies on the floatability of kimberlite minerals and diamonds.
Flotation in laboratory conditions can be used to simulate the industrial process of froth separation. When studying the interaction of apolar collecting agents with the surface of floatable minerals, a close correlation is observed between the extent of collecting agent attachment on minerals and their floatability [20]. The monomineral flotation method in a Hallimond tube is a widely recognized method for assessing the hydrophobicity of mineral surfaces and evaluating the potential for using collecting agents in industrial processes [21].
For flotation tests, a fraction of a size of −250 + 75 μm was separated from the crushed monomineral material by screening, from which a mineral sample weighing 150 mg was taken. The sample was kept in a 35 ml of aqueous phase for 60 minutes. Then, a regulating agent was added to the aqueous phase, the sample was kept for 5 minutes, and a collecting agent, F-5 bunker fuel, was added. The conditioning stage with the collecting agent lasted 1 minute. The collecting agent consumption was 2 µl (about 1.86 mg) per 35 ml of the aqueous phase, and the collecting agent concentration during conditioning was 52 mg/l. After conditioning, the sample with the aqueous phase was loaded into a flotation unit, topped up with the aqueous phase, and floated for 4 minutes at a total air flow rate of 50 ml. The temperature of the aqueous phase in the conditioning and flotation stages was 24 °C.
The results of the flotation tests showed that the addition of different classes of regulating agents has different effects on the floatability of diamonds and kimberlite minerals (Fig. 5).
A noticeable decrease in the recovery of diamonds and kimberlite minerals with the addition of a regulating agent is observed with CMC-75-V and Neonol AF-9-6. The character of the dependencies of diamond and kimberlite mineral recovery on the consumption of other regulating agents differs significantly. For instance, with NTPA addition, diamond recovery remains virtually unchanged, while the floatability of kimberlite minerals significantly decreases. PEG-1500 agent does not reduce the floatability of diamonds and has a relatively weak effect on the floatability of kimberlite minerals. OP-4 dispersing agent increases the floatability of diamonds and reduces the floatability of kimberlite minerals.
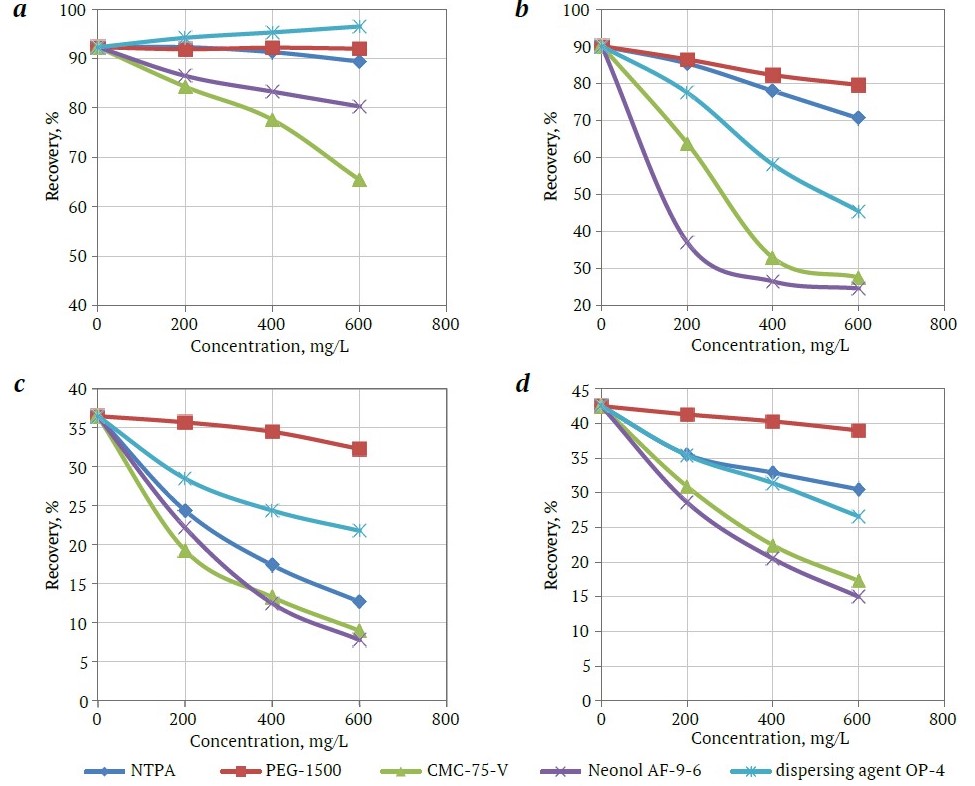
Fig. 5. The effect of regulating agents on floatability of diamond (a), talc (b), calcite (c), and muscovite (d): 1 – NTPA; 2 – PEG-1500; 3 – CMC-75-V; 4 – Neonol AF-9-6; 5 – OP-4 dispersing agent
Further studies were conducted on kimberlites differing in the degree of metamorphism and mineral composition: kimberlite sample 1 was represented mainly by primary minerals: olivine (55%), phlogopite and muscovite (8%), pyroxene (7%), calcite (aragonite) (6%), dolomite (2.2%), chromite (2%), iron sulfides (1.2%), titanomagnetite (1.0%), pyrope (0.3%), etc.; kimberlite sample 2 was represented by calcite (aragonite) (34%), olivine (22%), phlogopite and muscovite (6.5%), dolomite (6.4%), pyroxene (4.4%), chromite (2%), talc (2%), iron sulfides (1.1%), titanomagnetite (1.0%), pyrope (0.25%), etc.
Before flotation, a kimberlite sample was deslimed and a material with a particle size of −400 +180 mm went to flotation. The method of preparation and flotation of kimberlite corresponded to the method of flotation of diamonds and individual kimberlite minerals.
Based on the analysis of the data obtained during the flotation of diamonds and individual kimberlite minerals, a concentration of 200 mg/L of regulating agents was selected for kimberlite flotation tests (with an additional 50 mg/L for Neonol AF-9-6 and Oxypav A1218.30).
The results of the studies showed that it is feasible to suppress the floatablity of kimberlites when using the tested regulating agents. The agents Neolon AF-9-6, Oxypav A1218.30, IS-3, and Kamcel 600 most intensively reduce the floatability (yield) of kimberlites (Table 4).
Table 4
Influence of regulating agents on the flotation selectivity of diamonds and kimberlite
Regulating agent | Diamond recovery, % | Kimberlite yield into froth product, % | Selectivity criterion S, % | ||
Sample 1 | Sample 2 | Sample 1 | Sample 2 | ||
No additives | 92.3 | 3.77 | 4.94 | 87.40 | 85.88 |
NTPA | 92.5 | 2.04 | 2.51 | 89.85 | 89.24 |
OEDPA | 92.3 | 2.57 | 2.56 | 88.96 | 88.97 |
EDTA | 90.5 | 2.44 | 2.82 | 87.33 | 86.83 |
PEG-1500 | 91.9 | 5.65 | 4.86 | 84.56 | 85.58 |
STPP | 88.3 | 2.31 | 3.69 | 85.30 | 83.50 |
Neonol AF 9-6 | 86.3 | 0.67 | 1.92 | 85.43 | 83.80 |
Neonol AF 9-6 (50 mg/L) | 90.5 | 2.12 | 2.66 | 87.74 | 87.04 |
CMC 75-V | 88.5 | 2.16 | 1.79 | 85.69 | 86.17 |
IS-3 | 90.4 | 1.36 | 2.44 | 88.63 | 87.23 |
KAMCEL-600 | 88.8 | 1.97 | 2.43 | 86.24 | 85.64 |
OP-4 | 94.2 | 2.44 | 5.12 | 91.03 | 87.54 |
Oxypav A1218.30 | 84.3 | 1.33 | 1.48 | 82.57 | 82.38 |
Oxipav A1218.30 (50 mg/L) | 88.3 | 1.15 | 1.22 | 86.81 | 86.71 |
Triethanolamine | 88.3 | 3.4 | 3.87 | 83.88 | 83.27 |
Lactic acid | 89.2 | 3.2 | 3.99 | 85.04 | 84.01 |
However, since applying certain agents resulted in a decrease in diamond recovery, specialized criteria, such as selectivity criteria, must be applied to assess their effectiveness and suitability for use. The selectivity was evaluated using the equation proposed in [1] for the froth separation process:
S = ε – 1.3γ, (4)
where S is selectivity criterion; ε is diamond recovery; γ is yield (recovery) of kimberlite into concentrate.
The analysis of the calculated selectivity criterion showed that the highest selectivity of diamond flotation was achieved when using NTPA, OEDPA, IS-3, and OP-4 regulating agents (see Table 4).
Nevertheless, the feasibility of using some other studied regulating agents to increase the selectivity of flotation extraction of diamonds from kimberlites of various origins has been confirmed.
Thus, the studies conducted have established the feasibility of reducing the floatability of kimberlite minerals and kimberlite rock particles and improving the performance indicators of diamond-bearing kimberlite flotation using regulating agents of classes of alkylarylphosphonates, short-chain aliphatic amines, and complex agents. The use of agents with strong surfactant properties (ionic and nonionic nitrogen-containing short-chain polymers) will yield positive results when varying their consumption based on the data from the tests with reduced consumption of Neonol AF-9-8 and Oxipav A1218.30 agents.
Key findings
Based on the analysis of comprehensive physicochemical studies of the interaction of apolar collecting agents and regulating agents of various classes with the surface of diamond-bearing kimberlite minerals and the results of flotation studies, regulating agents have been justified and selected that ensure increased selectivity of the processes of diamond-bearing kimberlite beneficiation by froth and X-ray luminescence separation.
Visiometric analysis has determined that, among kimberlite minerals, talc, calcite, muscovite, pyrite, and phlogopite possess the highest adhesive activity in relation to apolar collecting agents (oil receptivity) and flotation response.
According to the measurements of the wetting contact angle, a clear trend has been identified towards a decrease in the adhesive activity of flotation-active kimberlite minerals in relation to the apolar collecting agent when using alkylarylphosphonates (NTPA, OEDPA), aliphatic alcohols (PEG-1500); bifunctionally modified CMC derivatives (CMC-75-V; Kamcel 600), ionogenic and non-ionogenic nitrogen-containing polymers (Neonol AF-9-6, Oxypav A1218.30), short-chain aliphatic amines (OP-4 dispersing agent), complex agents (IS-3 agent).
The calculations using the Dupré–Young equation (based on the experimentally obtained values of the wetting contact angle and surface tension at the interface between the organic collecting agent and the aqueous phase) showed that when regulating agents were added, the adhesion energy of the apolar collecting agent on kimberlite minerals, with the exception of talc, decreased by 2.5–6 times and reached values of 6–15 J/m2, comparable to the adhesion energy of the aqueous phase (5.7 J/m2). At the same time, under similar conditions, the adhesion energy of the collecting agent on diamonds decreased to the values of 17–27.5 J/m2 that caused the apolar collecting agent to adhere to diamonds stably. This fact predetermines the enhancement of the contrast in the flotation response of kimberlite minerals and diamonds due to applying the regulating agents and justifies the feasibility of increasing the selectivity of diamond flotation from kimberlites.
The results of the flotation studies on diamonds and kimberlites of different degrees of metamorphism confirmed the feasibility of increasing the selectivity of diamond flotation when using NTPA, OEDPA, IS-3, and OP-4 regulating agents. The listed regulating agents are recommended for testing in industrial froth separation processes in diamond-bearing kimberlite processing flowsheets.
References
1. Chanturiya V. A., Morozov V. V., Dvoichenkova G. P., et al. Optimizing composition and application conditions of agents for modifying spectral characteristics of diamonds in X-ray luminescence separation. Mining Science and Technology (Russia). 2023;8(4):313–326. https://doi.org/10.17073/2500-0632-2023-09-154
2. Morozov V. V., Chanturia, V. A., Dvoichenkova G. P., Chanturia E. L. Hydrophobic interactions in the diamond–organic liquid–inorganic luminophore system in modification of spectral and kinetic characteristics of diamonds. Journal of Mining Science. 2022;58(2):257–266. https://doi.org/10.1134/S1062739122020090
3. Kovalenko E. G., Babushkina A. L., Chut-Dy V. A. Application of multi-component collectors and selection of temperature modes for frother separation of diamond-bearing kimberlites. Gornyi Zhurnal. 2023;(12):51–62. (In Russ.) https://doi.org/10.17580/gzh.2023.12.12
4. Morozov V. V., Kovalenko E. G., Dvoychenkova G. P., et al. Current trends of improving the efficiency of froth separation of diamond-bearing kimberlites. Mining Science and Technology (Russia). 2024;9(2):134–145. https://doi.org/10.17073/2500-0632-2023-07-136
5. Morozov V. V., Chanturia V. A., Dvoichenkova G. P., et al. Selecting organic collectors for luminophore-bearing modifying agents to extract weakly fluorescent diamonds. Journal of Mining Science, 2023;59(2):292–301. https://doi.org/10.1134/S1062739123020126
6. Nguyen A. V., Schulze H. J. Colloidal science of flotation. New York: Marcel Dekker; 92004. 850 p.
7. Kondratyev S. A. Thermodynamic conditions for the presence of physically sorbed collectors on a mineral surface in an elementary act of flotation. Tsvetnye Metally. 2024;(7):118–136. https://doi.org/10.17580/tsm.2024.07.02
8. Zhang J., Kouznetsov D. L., Yu M., et al. Improving the separation of diamond from gangue minerals. Minerals Engineering. 2012;36–38:168–171. https://doi.org/10.1016/j.mineng.2012.03.015
9. Verkhoturov M. V., Amelin S. A., Konnova N. I. Diamond beneficiation. Mezhdunarodnyi Zhurnal Eksperimental'nogo Obrazovaniya. 2012;(2):61. (In Russ.)
10. Sergienko V. I., Perfilev A. V., Ksenik T. V., Yudakov A. A. Obtaining and application of hydrophobic adsorbents on the basis of aluminosilicates. Proceedings of the Kola Science Centre of the Russian Academy of Sciences. 2015;(5):108–112. (In Russ.)
11. Zemtsov Yu. V., Mazaev V. V. Current state of physicochemical enhanced oil recovery methods: a literature and patent review. Yekaterinburg: LLC «Izdatel'skie resheniya»; 2021. 239 p. (In Russ.)
12. Popov K. I., Kovaleva N. E., Rudakova G. Y., et al. Recent state-of-the-art of biodegradable scale inhibitors for cooling-water treatment applications (review). Thermal Engineering. 2016;63(2):122–129. https://doi.org/10.1134/S0040601516010092 (Orig. ver.: Popov K. I., Kovaleva N. E., Rudakova G. Y., et al. Recent state-of-the-art of biodegradable scale inhibitors for cooling-water treatment applications (review). Teploenergetika. 2016;(2):46–53. (In Russ.) https://doi.org/10.1134/S0040363616010094)
13. Siddiqui M. A. Q., et al. Current understanding of shale wettability: A review on contact angle measurements. Earth-Science Reviews. 2018;181:1–11. https://doi.org/10.1016/j.earscirev.2018.04.002
14. Dvoichenkova G. P., Kovalenko E. G., Timofeev A. S., Podkamennyi Yu. A. Enhanced efficiency of diamond foam separation after complex removal of hydrophilic slime coats from diamond surface. Mining Informational and Analytical Bulletin. 2022;(10):20–38. (In Russ.) https://doi.org/10.25018/0236_1493_2022_10_0_20
15. Bogatov M. V., Yudin P. E., Verevkin A. G., Berkov D. V. Effect of hydrophilicity, oleficity on formation of asphalt resin paraffin deposits. Petroleum Engineering. 2022;20(6):117–126. (In Russ.) https://doi.org/10.17122/ngdelo-2022-6-114-123
16. Samara H., Jaeger P. Experimental determination of wetting behavior under non-atmospheric conditions relevant to reservoirs: a practical guide. SN Applied Sciences. 2022;4:85. https://doi.org/10.1007/s42452-022-04963-8
17. Belhaj A. F., Elraies K. A., Mahmood S. M., et al. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: a review. Journal of Petroleum Exploration and Production Technology. 2020;10(1):125–137. https://doi.org/10.1007/s13202-019-0685-y9
18. Tret'yakov N. Yu., Panicheva L. P., Turnaeva E. A., et al. Synthesis of alkyl phosphates and investigation of their surface-active properties in an alkaline surfactant polymer system for enhanced oil recovery. Proceedings of Universities. Applied Chemistry and Biotechnology. 2021;11(1):147–158. (In Russ.) https://doi.org/10.21285/2227-2925-2021-11-1-147-158
19. Deryabin V. A., Farafontova E. P. Physical chemistry of disperse systems. Moscow: Yurayt; 2018. 86 p. (In Russ.)
20. Kondrat’ev S. A. Action of physisorbed collector in particle–bubble attachment. Journal of Mining Science. 2021;57(1):106–122. https://doi.org/10.1134/S1062739121010129 (Orig. ver.: Kondrat’ev S. A. Action of physisorbed collector in particle–bubble attachment. Fiziko-Texhnicheskiye Problemy Razrabbotki Poleznykh Iskopaemykh. 2021;(1):118–136. (In Russ.) https://doi.org/10.15372/FTPRPI20210112)
21. Błaszków A., Ratajczak T., Szyszka D. Flotation of hydrophobic minerals in Hallimond tube. Mining Science. 2024;31:219–227. https://doi.org/10.37190/msc243112
About the Authors
V. A. ChanturiyaRussian Federation
Valentin A. Chanturiya – Dr. Sci. (Eng.), Professor, Academician of the Russian Academy of Sciences
Moscow
Scopus ID 7004497128
ResearcherID J-9712-2014
V. V. Morozov
Russian Federation
Valeriy V. Morozov – Dr. Sci. (Eng.), Professor of the Department of General and Inorganic Chemistry, Research Institute of Comprehensive Exploitation of Mineral Resources of the Russian Academy of Sciences,
Moscow
Scopus ID 7402759618
E. L. Chanturiya
Russian Federation
Elena L. Chanturiya – Dr. Sci. (Eng.), Professor, Professor of the Department of Enrichment and Processing of Mineral Resources and Technogenic Raw Materials
Moscow
Scopus ID 57196009376
ResearcherID J-4214-2014
A. L. Samusev
Russian Federation
Andrey L. Samusev – Cand. Sci. (Eng.), Head of the Laboratory of Theory of Mineral Components Separation
Moscow
Scopus ID 54894913500
Supplementary files
Review
For citations:
Chanturiya V.A., Morozov V.V., Chanturiya E.L., Samusev A.L. Regulation of the oil receptivity of the surface of diamonds and kimberlite minerals using various classes of regulating agents. Mining Science and Technology (Russia). 2025;10(4):379–392. https://doi.org/10.17073/2500-0632-2025-09-454




































