Scroll to:
“Invisible” noble metals in carbonaceous rocks and beneficiation products: feasibility of detection and coarsening
https://doi.org/10.17073/2500-0632-2024-03-229
Abstract
The decrease in the quality of raw materials coming for processing requires involvement of refractory ores in processing, the refractoriness of which is caused by the presence of organic carbonaceous matter sorption-active in relation to dissolved noble metals and impregnation of fine noble metals in mineral-carriers. In this connection, the actual research line is the development of new technological solutions with the use of energy methods of action in order to reduce the losses of valuable components in beneficiation tailings. Treatment with ultra-high frequency electromagnetic radiation has a number of advantages, including rapid and selective heating due to the differences in the ability of minerals to absorb this radiation. Carbon-containing materials represented by carbonaceous flotation concentrate and model samples of activated carbon with adsorbed silver were taken as the research subjects. Using the model samples as an example, the necessity of using magnetite to achieve coarsening fine silver particles during ultra-high frequency treatment was substantiated. The formation of active centers of local heating during the treatment in the points of magnetite addition was confirmed. The necessary content of magnetite of 10% for coarsening fine silver to spherical aggregates, the average size of which was 20–40 microns, was substantiated. Coarsening noble metal particles to sizes of 20–50 microns in treated carbonaceous concentrates containing silver and gold was achieved, when the substantiated amount of magnetite was added. Coarsened particles (aggregates) of noble metals can be recovered using traditional beneficiation methods.
Keywords
For citations:
Aleksandrova Т.N., Afanasova A.V., Aburova V.A. “Invisible” noble metals in carbonaceous rocks and beneficiation products: feasibility of detection and coarsening. Mining Science and Technology (Russia). 2024;9(3):231-242. https://doi.org/10.17073/2500-0632-2024-03-229
“Invisible” noble metals in carbonaceous rocks and beneficiation products: feasibility of detection and coarsening
Introduction
The development of technologies for processing of strategic raw materials is necessary to maintain the world economy at the current level in connection with the depletion of mineral resource base. Worsening the quality of gold- and silver-containing raw materials, involvement of lean and refractory ores in processing owes the actualization of scientific research aimed at increasing the recovery of valuable components into concentrates [1]. The analysis of existing studies has shown that some of the topical areas in the field of mineral beneficiation are increasing the efficiency of disintegration of mineral raw materials [2, 3], application of machine vision technology at various stages of beneficiation, synthesis of new flotation reagents [4–6], and the development of new reagent regimes [7–9].
The refractoriness of gold-bearing ores can be caused both by the presence of substances sorption-active in relation to dissolved gold and inclusions of fine noble metals in mineral-carriers, mainly in such minerals as pyrite, arsenopyrite, galena, etc. Ores in which both these features are present are referred to ores of double refractoriness. “Invisible” gold is referred to submicroscopic gold particles that are 1–100 nm in size and are not detected using optical or electron microscopy [10]. The presence of “invisible” gold and silver in ores complicates the selection of flow charts for their processing and owes the necessity for the development of new process solutions and improvement of existing ones.
The carbonaceous material contained in the double-refractory ores contaminates the concentrates produced and results in significant losses of valuable components at the stage of metallurgical processing. The actual task of the existing research is to involve in processing carbonaceous products, going to tailings at most of the processing plants, in order to reduce losses of valuable components such as gold and silver [11–13].
In addition to native metal, silver in ores can be represented by dispersed inclusions in the host minerals, either as metallic or chemically bound silver. Chemically bound silver refers to various silver sulfides, for example, Ag2S. The presence of fine silver associated with sulfide minerals, as well as in the case of gold, complicates its recovery at the cyanidation stage [14]. The recovery requires destruction of the matrix of the host minerals in order to provide the contact of reagents with the noble metal to dissolve it.
To date, there is a large amount of researches aimed at finding process solutions for the processing of refractory raw materials [15–17]. For double refractory ores, pretreatment to reduce the refractoriness of gold-bearing ores is required. The most common pretreatment methods are roasting, chlorination, pressure oxidation, bioleaching, and Albion technology. However, in addition to the traditional methods, research into the feasibility of using energy-based methods of action has become widespread [18, 19]. Ultra-high frequency (UHF) treatment is one of the promising methods whose advantages include fast and selective heating [20–22]. The selectivity of heating minerals is due to the differences in their heating rate, which in turn is connected with specific heat capacity, specific thermal conductivity, and relative dielectric constant. Recent researches with the use of microwave treatment are aimed at increasing the efficiency of disintegration of mineral raw materials [23-25], studying the feasibility of using microwaves for sorting ores [26], reducing the content of harmful impurities in ores [27], studying the effect on surface properties and flotability of minerals [28], involvement of cyanide [29] and sulfide [30] tailings in the treatment. The melting temperature of “invisible” noble metals is much lower than the melting temperature of visible particles that predetermines the feasibility of their coarsening in the process of energy actions. For example, for Au, the melting point of a 1.6 nm diameter cluster is 257.85 °C [33], while that of a 1.9 nm diameter cluster is 318.85 °C [34]. For Ag, the melting point of a 4 nm diameter cluster is 449.85 °C [5], while that of a 5 nm diameter cluster is 509.85 °C [34]. Carbonaceous flotation products, as a rule, contain insignificant amounts of ore minerals, which requires sufficiently long time for microwave treatment in order to coarsen the particles. In this regard, the addition of magnetite in the process of treatment allows the creation of local heating centers. Table 1 systematizes the data of dependence of the achieved maximum heating temperature on the treatment time for sulfide mineral-concentrators of gold and products of their destruction after roasting.
Analysis of historical studies confirms the fact that magnetite is more active in relation to ultra-high frequency electromagnetic radiation and, in comparison with other minerals, reaches significantly higher heating temperatures. The low melting temperature of silver nanoclusters confirms the prospect of investigating the feasibility of their coarsening, since it is lower than the magnetite treatment heating temperature.
Thus, the purpose of this study was to reveal the mechanism of coarsening fine silver in the course of microwave treatment with the addition of magnetite using model samples and to justify the required content of magnetite to confirm the feasibility of silver coarsening in the course of microwave treatment of carbonaceous flotation concentrate samples to reduce losses of valuable components in tailings.
Table 1
Summary data on dependence of maximum microwave heating temperature on treatment time
| No. | Mineral | Treatment time, min | Maximum temperature, °C | Reference |
| 1 | Magnetite | 2.75 | 1258 | [31] |
| 2 | Pyrite | 6.75 | 1019 | [31] |
| 3 | Pyrrhotite | 1.75 | 886 | [31] |
| 4 | Arsenopyrite | 1.0 | 723 | [31] |
| 5 | Hematite | 7.0 | 182 | [31] |
Research Materials and Techniques
1. Characterization of research subjects
The following carbon-containing materials were chosen as the research subjects:
- carbonaceous flotation concentrate (produced by carbonaceous flotation from refractory sulphide gold-bearing ore);
- model samples of activated carbon (after adsorption of silver).
The source of silver in the model sample was silver leaf, containing 99.9% Ag. Preparation of the model samples included: grinding of activated carbon, preparation of leaching solution, transition of silver leaf to liquid phase for two days (leaching), its subsequent contacting with activated carbon for three days. The leaching reagent is a mixture of iodine complex, amino acids, sodium chloride, urea, ammonium chloride, and sodium carbonate. After contacting the noble metal with activated carbon, filtration and drying of the obtained cake was carried out for subsequent microwave treatment.
The initial carbonaceous gold-bearing ore is characterized by the presence of fine inclusions of gold and insignificant amount of silver in the mineral-concentrators, such as pyrite and arsenopyrite, as well as organic carbonaceous matter sorption-active in relation to dissolved gold. The main valuable component is gold, the grade of which in the initial ore is 5.99 ± 0.29 g/t. Silver is a by-product, the grade of which is 0.29 ± 0.1 g/t.
2. Flotation beneficiation experiments
Separation of carbonaceous flotation concentrate from refractory gold-bearing ore was carried out using pneumomechanical flotation machine Flotation Bench Test Machine of Laarmann company with cell volume of 1.5 liters. The initial ore was subjected to grinding to particle size of 60% passing 71 μm, after which carbonaceous flotation was carried out with the addition of oxal frothing agent, the consumption of which was 85 g/t. The results of studying the obtained flotation products are presented in Table 2.
The obtained carbonaceous flotation concentrate after drying was subjected to microwave treatment, while the tailings of carbonaceous flotation went for additional grinding and subsequent sulfide flotation.
Table 2
Results of research of refractory gold-bearing ore flotation products
| Product Designation | Yield, % | Content [Grade], % [*g/t] | Recovery, % | ||||||
| Au* | Ag* | Corg | Stot | Au | Ag | Corg | Stot | ||
| Carbonaceous flotation concentrate | 2.42 | 2.91 | 0.12 | 27.14 | 0.95 | 1.19 | 0.94 | 40.54 | 1.36 |
| Carbonaceous flotation tailings | 97.58 | 5.99 | 0.31 | 0.99 | 1.71 | 98.81 | 99.06 | 59.46 | 98.64 |
| Initial ore | 100.00 | 5.92 | 0.31 | 1.62 | 1.69 | 100.00 | 100.00 | 100.00 | 100.00 |
3. Microwave treatment
Microwave treatment of carbon-containing materials without and with the addition of different amount of magnetite (3, 5, 10, 15% by weight of a sample) was carried out using a Sineo UWave-2000 microwave oven, the range of possible setting of power in which was 100–1000 W. The coarseness of the added magnetite was −50 μm.
4. Scanning electron microscopy
In order to confirm coarsening fine silver in model samples and carbonaceous flotation concentrate after microwave treatment with the addition of magnetite to create active centers of local heating, the study of the samples before and after the treatment was carried out using a Vega 3 LMH scanning electron microscope, combined with a system of X-ray energy dispersive microanalysis Oxford Instruments INCA Energy 250/X-max 20. The fine silver coarsening study was carried out with the determination of sizes of a minimum of 200 particles in each of the samples obtained. The carbonization of the samples was carried out using a Q150R E sputtering apparatus manufactured by Quorum Technologies Ltd.
5. Microwave heating temperature
Measuring the achieved temperature of microwave heating of samples was carried out using a FinePower DIN21H laser pyrometer. The measuring temperature range was −50 to 1,100 °C with an accuracy of ±2%.
Findings and Discussion
1. Processing of silver-containing model samples without magnetite addition
In order to confirm the feasibility of coarsening fine silver in the course of microwave treatment, an initial model sample with adsorbed silver before treatment was studied for comparison. Figure 1 shows the results of the sample study before treatment using scanning electron microscopy. Table 3 shows the findings of the elemental composition study for the spectrum shown in Fig. 1.
Based on the results obtained, the absence of visible silver was revealed, which was confirmed by the results of the elemental composition study, thus confirming its “invisibility”.
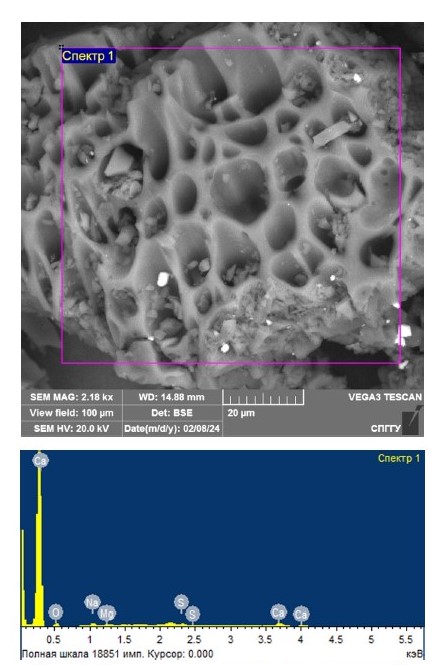
Fig. 1. Results of investigation of the initial model sample before microwave treatment using scanning electron microscopy
Table 3
The results of the study of elemental composition for the spectra presented in Fig. 1
| Spectrum number | Content, weight % | ||||
| O | Na | Mg | S | Ca | |
| Spectrum 1 | 73.67 | 8.21 | 2.74 | 2.56 | 12.82 |
In order to confirm the necessity of adding magnetite to silver-containing model samples at microwave treatment for coarsening fine silver, the studies were carried out on model samples without magnetite addition. Figure 2 shows the results of scanning electron microscopy examination of the treated samples without magnetite addition. The results of the study of the elemental composition for the spectra in the studied samples are shown in Table 4.
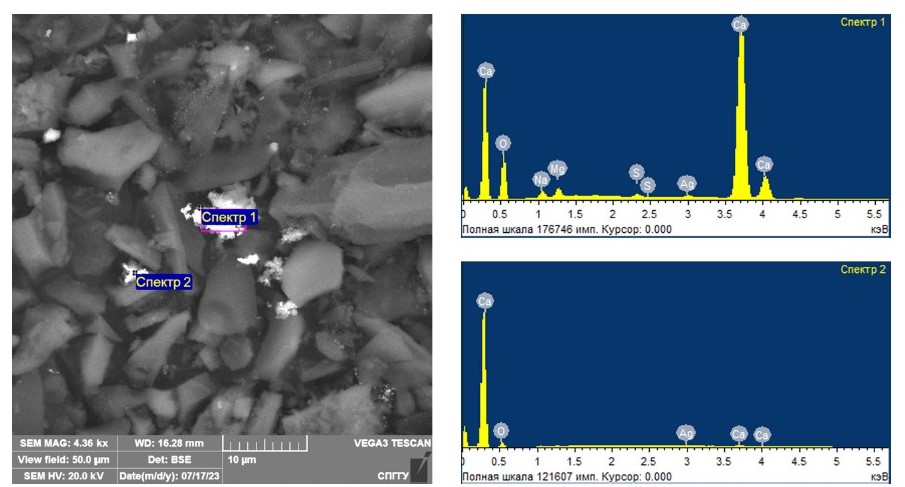
Fig. 2. Results of scanning electron microscopy study of model samples with coarsened silver after microwave treatment without magnetite addition
Table 4
The results of the study of elemental composition for the spectra presented in Fig. 2
| Spectrum number | Content, weight % | |||||
| O | Na | Mg | S | Ca | Ag | |
| Spectrum 1 | 49.67 | 1.50 | 1.42 | 0.38 | 45.78 | 1.25 |
| Spectrum 2 | 76.10 | – | – | – | 16.16 | 7.74 |
The analysis of the obtained data allowed confirming the feasibility of coarsening fine silver particles using microwave treatment without addition of magnetite up to the average size equal to 5–10 microns. Despite the fact that coarsening is achieved at the treatment without the addition of magnetite, the coarseness achieved in these studies does not allow extracting coarsened particles by traditional beneficiation methods. This fact predetermines the need to study the effect of the addition of different magnetite amounts to the studied model samples on coarsening fine silver particles.
2. Processing of silver-containing model samples with magnetite addition
The studies aimed at establishing the necessary magnetite content to achieve maximum coarsening fine silver in the course of microwave treatment were carried out using silver-containing model samples. The magnetite contents were chosen at 3, 5, 10, and 15%. To confirm the generation of active centers of local heating in the course of microwave treatment of the model samples with the addition of magnetite, heating temperatures were measured over the whole area of the treated samples using a laser pyrometer. Fig. 3 shows the temperature mapping using an example of microwave treatment of a silver-containing model sample with 10% magnetite with sampling points for scanning electron microscopy study. The microwave heating temperature was measured immediately after the treatment. The results of the elemental composition study for the spectra shown in Fig. 3 are presented in Table 5.
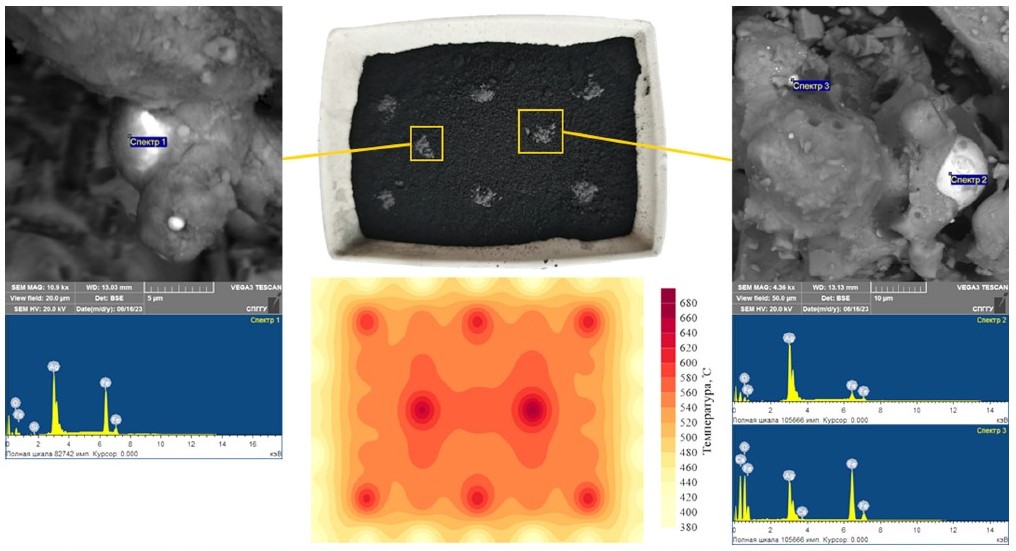
Fig. 3. Temperature mapping of silver-containing model sample at microwave treatment with addition of 10% magnetite with the results of sample examination at selected sampling points using scanning electron microscopy (gray – magnetite; black – model sample)
Table 5
The results of the elemental composition study for the spectra presented in Fig. 3
| Spectrum number | Content, weight % | ||||
| O | Si | Ca | Fe | Ag | |
| Spectrum 1 | 10.17 | 0.12 | – | 42.22 | 47.48 |
| Spectrum 2 | 11.69 | 0.11 | – | 11.51 | 76.69 |
| Spectrum 3 | 35.06 | – | 0.24 | 41.40 | 23.30 |
The interpretation of the obtained data allows confirming arising active centers of local heating in the course of microwave treatment when magnetite was added to the model samples. In Fig. 3, the temperature peaks at the magnetite addition locations, which reach about 600 °C and higher, are clearly visible. At the same time, the heating temperature of the model sample itself is 540–560 °C. The presence of magnetite is confirmed by the results of the elemental composition study.
The treated silver-containing model samples with magnetite addition were studied using a scanning electron microscope. Figs. 4 and 5 show the results of the study of these samples with magnetite content of 3, 5, 10, and 15%, respectively. The results of the study of elemental composition for the spectra shown in Figs. 4 and 5 are shown in Table 6.
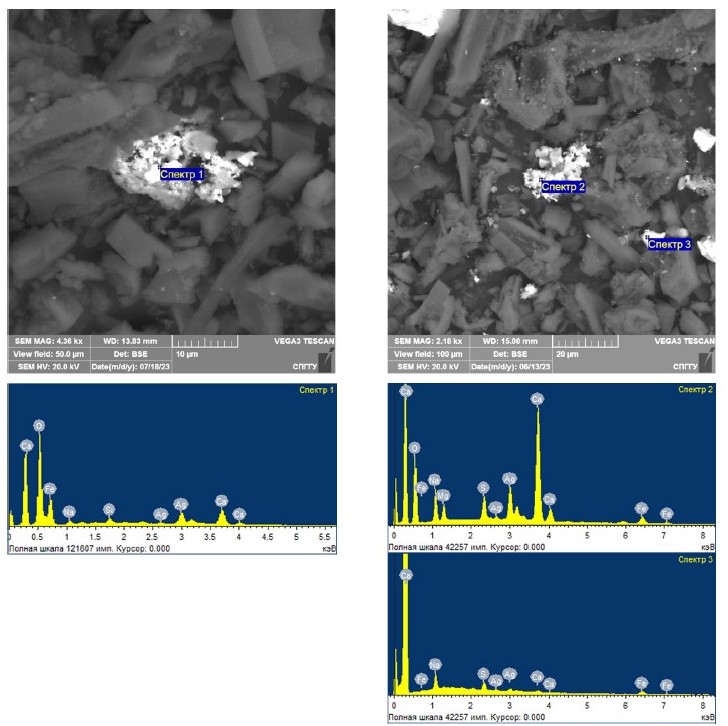
Fig. 4. Results of scanning electron microscopy study of model samples with coarsened silver after microwave treatment with addition of 3 (left) and 5 (right) % magnetite
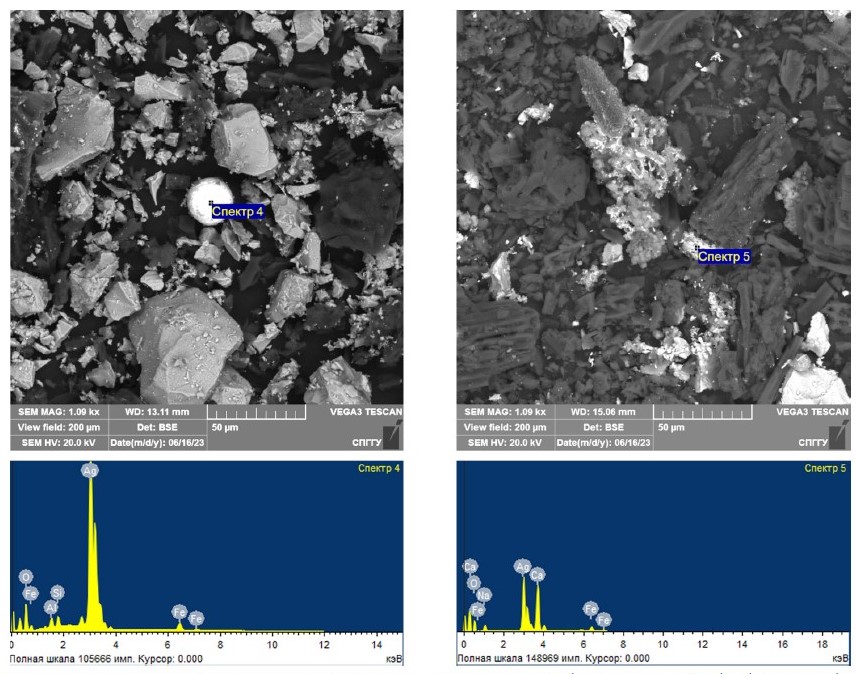
Fig. 5. Results of scanning electron microscopy study of model samples with coarsened silver after microwave treatment with addition of 10 (left) and 15 (right) % magnetite
Table 6
The results of the elemental composition study for the spectra presented in Figs. 4 и 5
| Spectrum number | Content, weight % | ||||||||
| O | Na | Mg | Al | Si | S | Ca | Fe | Ag | |
| Spectrum 1 | 46.79 | 1.14 | – | – | 0.70 | – | 3.30 | 42.73 | 5.34 |
| Spectrum 2 | 49.52 | 6.06 | 2.04 | – | – | 2.65 | 22.60 | 3.74 | 13.39 |
| Spectrum 3 | – | 33.68 | – | – | – | 14.74 | 5.79 | 26.84 | 18.95 |
| Spectrum 4 | 20.02 | – | – | 0.94 | 0.98 | – | – | 2.97 | 75.09 |
| Spectrum 5 | 21.32 | 2.92 | – | – | – | – | 21.63 | 5.24 | 48.89 |
The interpretation of the obtained data allows to confirm coarsening fine silver at addition of magnetite as a result of microwave treatment to the particle sizes exceeding the particle size formed in the samples without magnetite, as well as the increase of silver content in the coarsened particles. However, it is worth noting that at a magnetite content of 10%, if compared to the coarsened gold presented earlier in [36], spherical-shaped silver particles with an average size of 20–40 μm were found in the silver-containing model samples. This is because the melting point of silver is much lower than that of gold.
The obtained data on average and maximum sizes of coarsened noble metal particles (aggregates) depending on the magnetite content are presented in Table 7. Besides, Table 7 presents the degree of coarsening measured as the ratio of the average size of the coarsened silver particles to the estimated coarseness of the “invisible” silver.
Table 7
Results of the study of the effect of magnetite content on coarsening fine silver in model samples with and without microwave treatment
| Parameter | Value | |||||
| Magnetite content, % | 0 (no treatment) | 0 | 3 | 5 | 10 | 15 |
| Average size of coarsened fine silver, µm | 1–100 nm | 5–10 | 10–15 | 10–20 | 20–40 | 10–15 |
| Maximum size of coarsened fine silver, µm | 39.0 | 43.0 | 92.3 | 123.1 | 102.0 | |
| Coarsening degree | 75–7500 | 125–12500 | 150–15000 | 300–30000 | 125–12500 | |
| Average fineness of magnetite, µm | 10–15 | |||||
| Maximum fineness of magnetite, µm | 49.0 | |||||
Based on the obtained data analysis, it can be concluded that the addition of magnetite, which forms active centers of local heating, contributes to coarsening fine silver particles in the course of microwave treatment, since the treatment of magnetite-free model samples allowed to coarsen the noble metal particles to smaller sizes if compared with the samples with magnetite. The obtained results allow justifying the necessary magnetite content of 10% to achieve maximum coarsening fine silver at microwave exposure to a coarseness of 20–40 μm. The formation of spherical silver particles can be explained by the lower melting point of silver compared to gold.
3. Treatment of carbonaceous flotation concentrate with addition of substantiated amount of magnetite
The feasibility of silver coarsening with the addition of the previously substantiated magnetite amount in the course of microwave treatment was investigated on carbonaceous flotation concentrates. In [37], the feasibility of coarsening fine Au particles using 10% magnetite at microwave treatment of carbonaceous concentrate was confirmed. Fig. 6 presents the results of a scanning electron microscope study of the treated carbonaceous concentrate, confirming coarsening silver in the course of microwave treatment when added with magnetite in the amount of 10% of the sample weight. The elemental composition of the coarsened particles is presented in Table 8.
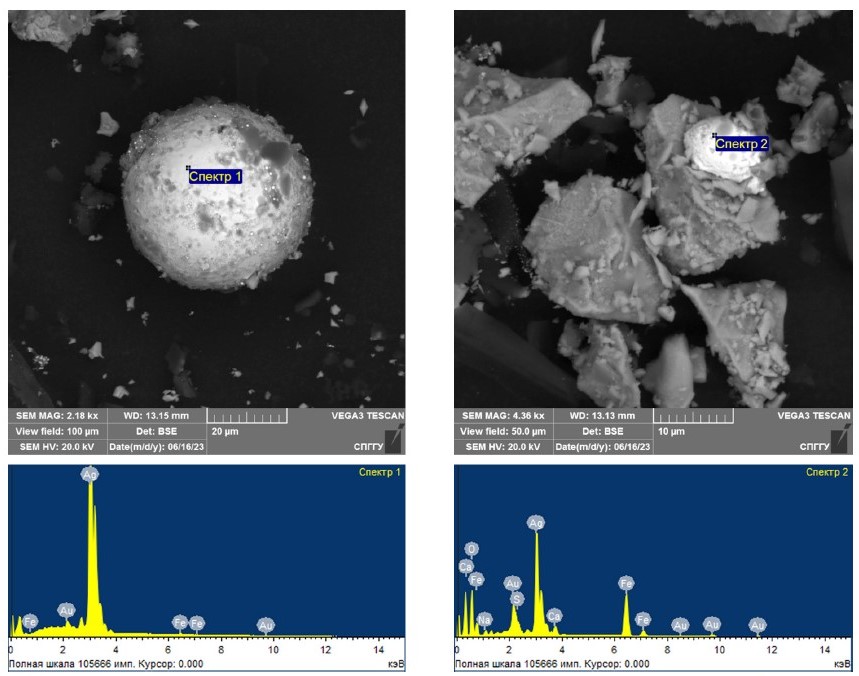
Fig. 6. Results of the study of treated carbonaceous concentrates with addition of 10% magnetite using scanning electron microscopy
Table 8
The results of the elemental composition study for the spectra presented in Fig. 6
| Spectrum number | Content, weight % | ||||||
| O | Na | S | Ca | Fe | Au | Ag | |
| Spectrum 1 | – | – | – | – | 0.96 | 3.84 | 95.20 |
| Spectrum 2 | 26.59 | 1.12 | 0.50 | 1.48 | 20.46 | 12.83 | 37.02 |
The interpretation of the obtained results allows to confirm coarsening silver particles in the course of microwave treatment of carbonaceous flotation concentrate added with 10% magnetite. The size of coarsened particles, which are characterized by spherical shape, after treatment is 20–50 microns. It is worth noting that, besides silver, characteristic peaks of gold were found in the coarsened aggregates according to the results of the study of the elemental composition. This indicates that silver was coarsened together with gold.
Microwave treatment contributes to increasing the temperature of the model sample, the main part of which is represented by activated carbon. When it is ignited in an oxidizing environment, the following phenomena occur:
- CO2 formation;
- melting of adsorbed fine silver particles after combustion of a part of activated carbon and magnetite heating;
- coarsening due to silver movement into additional pores formed.
Fig. 7 shows a schematic representation of the mechanism of fine silver coarsening in the course of microwave treatment.
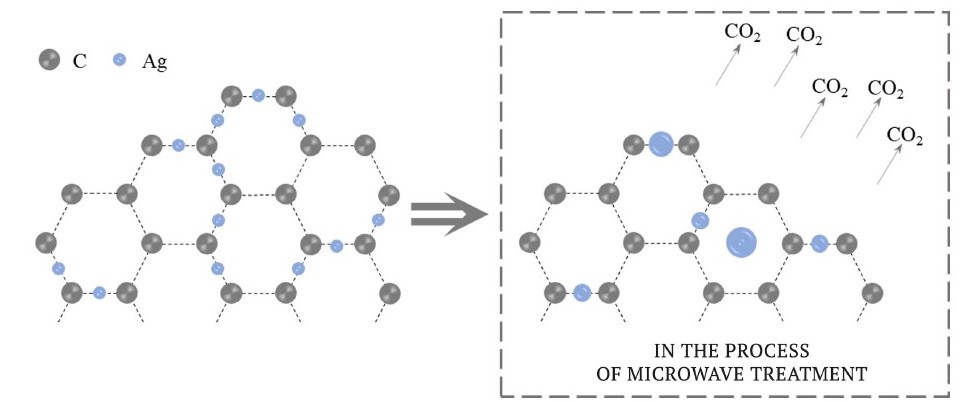
Fig. 7. Schematic representation of fine silver coarsening in the course of microwave treatment
Fine silver coarsening is confirmed by the results of scanning electron microscopy examination of the treated silver-containing model samples. In the figures presented in this paper, the coarsened particles are observed both free (not tied with activated carbon and magnetite) and at the initial stage of coarsening directly on the activated carbon surface itself.
The results of the microwave treatment of carbonaceous flotation concentrate with the addition of 10% magnetite also confirmed the feasibility of fine silver coarsening. The achieved coarseness and the content (grade) of gold and silver in the coarsened spherical particles makes it possible to further extract them and, as a consequence, to reduce losses of valuable components with tailings. Centrifugal concentration can be considered as a potential method for the extraction of the coarsened particles [38].
Conclusion
The findings of the studies on microwave treatment of silver-containing model samples allowed substantiating the necessity of adding magnetite for fine silver coarsening. Based on the temperature mapping, the formation, in the course of the treatment, of active centers of local heating in the locations of magnetite addition was confirmed. The fine silver coarsening to spherical silver particles, whose average size was 20–40 μm, was achieved at a microwave oven power of 1.0 kW and a treatment time of 3 min with 10% magnetite added. It was revealed that at similar microwave oven power and treatment time of 5 min, with 10% magnetite added, the greatest sizes of coarsened particles containing gold and silver equal to 20–50 μm were achieved. The microwave treatment findings showed that the silver coarsening occurs forming a gravity-extractable form of silver that allowed further investigation of its recovery using gravity concentration methods.
References
1. Fedotov P. K., Senchenko A. E., Fedotov K. V., Burdonov A. E. Studies of enrichment of sulfide and oxidized ores of gold deposits of the Aldan shield. Journal of Mining Institute. 2020;242:218–227. https://doi.org/10.31897/PMI.2020.2.218
2. Efimov D. A., Gospodarikov A. P. Technical and technological aspects of the use of Reuleaux triangular profile rolls in crushing units in the ore processing plant. Mining Informational and Analytical Bulletin. 2022;(10–2):117–126. (In Russ.) https://doi.org/10.25018/0236_1493_2022_102_0_117
3. Nikolaeva N. V., Kallaev I. T. Features of copper–molybdenum ore grinding. Mining Informational and Analytical Bulletin. 2024;(1):52–66. (In Russ.) https://doi.org/10.25018/0236_1493_2024_1_0_52
4. Lopéz R., Jordão H., Hartmann R. et al. Study of butyl-amine nanocrystal cellulose in the flotation of complex sulphide ores. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2019;579:123655. https://doi.org/10.1016/j.colsurfa.2019.123655
5. Chanturia V. A., Matveeva T. N., Ivanova T. A., Getman V. V. Mechanism of interaction of cloud point polymers with platinum and gold in flotation of finely disseminated precious metal ores. Mineral Processing and Extractive Metallurgy Review. 2016;37(3):187–195. https://doi.org/10.1080/08827508.2016.1168416
6. Matveeva T. N., Gromova N. K., Ivanova T. A., Chanturia V. A. Physicochemical effect of modified diethyldithiocarbamate on the surface of auriferous sulfide minerals in noble metal ore flotation. Journal of Mining Science. 2013;49(5):803–810. https://doi.org/10.1134/S1062739149050158
7. Owusu C., Agorhom E. A., Fosu S., Budu-Arthur E. Adsorption studies of sulphidic refractory gold ore. Powder Technology. 2020;375:310–316. https://doi.org/10.1016/j.powtec.2020.07.063
8. Yakovleva T. A., Romashev A. O., Mashevsky G. N. Digital technologies for optimizing the dosing of flotation reagents during flotation of non-ferrous metal ores. Mining Informational and Analytical Bulletin. 2022;(6-2):175–188. (In Russ.) https://doi.org/10.25018/0236_1493_2022_62_0_175
9. Aleksandrova T. N., Prokhorova E. O. Modification of properties of rock-forming minerals during flotation. Mining Informational and Analytical Bulletin. 2023;(12):123–138. (In Russ.) https://doi.org/10.25018/0236_1493_2023_12_0_123
10. Zakharov B. A., Meretukov M. A. Gold: refractory ores. Moscow: Ruda i Metally Publ. House; 2013. 452 p. (In Russ.)
11. Shumilova L. V., Kostikova O. S. Sulfidization of silver-polymetallic ores of “Goltsovoe” deposit for decreasing loss of silver in mill tailings. Journal of Mining Institute. 2018;230:160–166. https://doi.org/10.25515/PMI.2018.2.160
12. Rasskazova A. V., Sekisov A. G., Burdonov A. E. Activation leaching of difficult primary ore at Malmyzh deposit. Mining Informational and Analytical Bulletin. 2023;(1):130–141. (In Russ.) https://doi.org/10.25018/0236_1493_2023_1_0_130
13. Lavrik A. V., Konareva T. G., Rasskazova A. V. Recovery of submicron encapsulated gold from rebellious ore of Delken deposit. Mining Informational and Analytical Bulletin. 2021;(12–1):121–128. (In Russ.) https://doi.org/10.25018/0236_1493_2021_121_0_121
14. Lodeyshchikov V. V. Technology of gold and silver extraction from refractory ores: in 2 vol. v. 1, Irkutsk: JSC Irgiredmet Publ.; 1999. 452 p. (In Russ.)
15. Grigoreva V. A., Boduen A. Ya. Prospects for refractory gold-sulfide ore processing. Izvestiya. Non-Ferrous Metallurgy. 2023;(6):22–34. https://doi.org/10.17073/0021-3438-2023-6-22-34
16. Petrov G. V., Gordeev D. V., Bekirova V. R. Comparison of methods for enhancing gold recovery from double refractory concentrates using the technology of autoclave oxidation. iPolytech Journal. 2023;27(4):809–820. (In Russ.) https://doi.org/10.21285/1814-3520-2023-4-809-820
17. Ivanik S. A., Ilyukhin D. A. Flotation extraction of elemental sulfur from gold-bearing cakes. Journal of Mining Institute. 2020;242:202–208. https://doi.org/10.31897/PMI.2020.2.202
18. Amankwah R. K., Pickles C. A. Microwave roasting of a carbonaceous sulphidic gold concentrate. Minerals Engineering. 2009;22(13):1095–1101. https://doi.org/10.1016/j.mineng.2009.02.012
19. Chanturiya V. A., Bunin I. Z. Advances in Pulsed Power Mineral Processing Technologies. Minerals. 2022;12(9):1177. https://doi.org/10.3390/min12091177
20. Wei W., Shao Z., Zhang Y. et al. Fundamentals and applications of microwave energy in rock and concrete processing – a review. Applied Thermal Engineering. 2019;157:113751. https://doi.org/10.1016/j.applthermaleng.2019.113751
21. Haque K. E. Microwave energy for mineral treatment processes – a brief review. International Journal of Mineral Processing. 1999;57(1):1–24. https://doi.org/10.1016/s0301-7516(99)00009-5
22. Amini A., Latifi M., Chaouki J. Electrification of Materials Processing via Microwave Irradiation: A Review of Mechanism and Applications. Applied Thermal Engineering. 2021;193:117003. https://doi.org/10.1016/j.applthermaleng.2021.117003
23. Pressacco M., Kangas J. J. J., Saksala T. Numerical modelling of microwave irradiated rock fracture. Minerals Engineering. 2023;203:108318. https://doi.org/10.1016/j.mineng.2023.108318
24. Shadi A., Ahmadihosseini A., Rabiei M. et al. Numerical and experimental analysis of fully coupled electromagnetic and thermal phenomena in microwave heating of rocks. Minerals Engineering. 2022;178:107406. https://doi.org/10.1016/j.mineng.2022.107406
25. Qin L., Chen G., Xu G. et al. Microscopic liberation mechanisms of oolitic iron ore under microwave irradiation and optimization of irradiation parameters. Minerals Engineering. 2022;178:107402. https://doi.org/10.1016/j.mineng.2022.107402
26. Duan B., Bobicki E. R., Hum S. V. Application of microwave imaging in sensor-based ore sorting. Minerals Engineering. 2023;202:108303. https://doi.org/10.1016/j.mineng.2023.108303
27. Siva L. M. da, Nascimento M., Oliveira E. M. de et al. Evaluation of the diffusional coefficient in the acid baking process using microwave energy to reduce phosphorus content in iron ore particles. Minerals Engineering. 2020;157:106541. https://doi.org/10.1016/j.mineng.2020.106541
28. Silva G. R. da, Espiritu E. R. L., Mohammadi-Jam S., Waters K. E. Surface characterization of microwave-treated chalcopyrite. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2018;555:407–417. https://doi.org/10.1016/j.colsurfa.2018.06.078
29. Li H., Long H., Zhang L. et al. Effectiveness of microwave-assisted thermal treatment in the extraction of gold in cyanide tailings. Journal of Hazardous Materials. 2020;384:121456. https://doi.org/10.1016/j.jhazmat.2019.121456
30. Kamariah N., Kalebic D., Xanthopoulos P. et al. Conventional versus microwave-assisted roasting of sulfidic tailings: mineralogical transformation and metal leaching behavior. Minerals Engineering. 2022;183:107587. https://doi.org/10.1016/j.mineng.2022.107587
31. Walkiewicz J. W., Kazonich G., McGill S. L. Microwave heating characteristics of selected minerals and compounds. Minerals & metallurgical processing. 1988;5:39–42. https://doi.org/10.1007/BF03449501
32. Farahat M., Elmahdy A. M., Hirajima T. Influence of microwave radiation on the magnetic properties of molybdenite and arsenopyrite. Powder Technology. 2017;315:276–281. https://doi.org/10.1016/j.powtec.2017.04.023
33. Golovenko Zh. V., Gafner S. L., Gafner Yu. Ya. Computer analysis of the structural properties of gold nanoclusters. Fundamental Problems of Radio-Electronic Instrument Making. 2010;7(2):11–16. (In Russ.)
34. Golovenko Zh. V., Gafner S. L., Gafner Yu. Ya. Investigation of structural states of gold nanoclusters by molecular dynamics method. Fundamental Problems of Radio-Electronic Instrument Making. 2008;8(2):83–86. (In Russ.)
35. Samsonov V. M., Sdobnyakov N. Y., Myasnichenko V. S. et al. A comparative analysis of the size dependence of the melting and crystallization temperatures in silver nanoparticles via the molecular dynamics and Monte-Carlo methods. Journal of Surface Investigation: X-Ray, Synchrotron and Neutron Techniques. 2018;12(6):1206–1209. https://doi.org/10.1134/S1027451018050671 (Orig. ver.: Samsonov V. M., Sdobnyakov N. Y., Myasnichenko V. S. et al. A comparative analysis of the size dependence of the melting and crystallization temperatures in silver nanoparticles via the molecular dynamics and Monte-Carlo methods. Poverkhnost’. Rentgenovskie, Sinkhrotronnye i Neitronnye Issledovaniya. 2018;(12):65–69. https://doi.org/10.1134/S0207352818120168)
36. Aleksandrova T. N., Nikolaeva N. V., Afanasova A. V. et al. Extraction of low-dimensional structures of noble and rare metals from carbonaceous ores using low-temperature and energy impacts at succeeding stages of raw material transformation. Minerals. 2023;13(1):84. https://doi.org/10.3390/min13010084
37. Afanasova A. V., Aburova V. A. Growth of low-dimensional structure noble metals in carbonaceous materials under microwave treatment. Mining Informational and Analytical Bulletin. 2024;(1):20–35. (In Russ.) https://doi.org/10.25018/0236_1493_2024_1_0_20
38. Amdur A. M., Fedorov S. A., Matushkina A. N. Extraction of gold from definitely processing ores and technogenic waste by their high-temperature treatment and subsequent centrifugal separation. Mining Informational and Analytical Bulletin. 2022;(11-1):95–106. (In Russ.) https://doi.org/10.25018/0236_1493_2022_111_0_95
About the Authors
Т. N. AleksandrovaRussian Federation
Tayana N. Aleksandrova – Dr. Sci. (Eng.), Professor, Corresponding Member of the Russian Academy of Sciences
Scopus ID 57216873316, ResearcherID A-5418-2014
St. Petersburg
A. V. Afanasova
Russian Federation
Anastasia V. Afanasova – Cand. Sci. (Eng.), Associate Professor
Scopus ID 57188630049, ResearcherID AAH-4333-2019
St. Petersburg
V. A. Aburova
Russian Federation
Valeria A. Aburova – PhD-Student
Scopus ID 57503048800
St. Petersburg
Supplementary files
Review
For citations:
Aleksandrova Т.N., Afanasova A.V., Aburova V.A. “Invisible” noble metals in carbonaceous rocks and beneficiation products: feasibility of detection and coarsening. Mining Science and Technology (Russia). 2024;9(3):231-242. https://doi.org/10.17073/2500-0632-2024-03-229




































