Scroll to:
Enhancing flotation beneficiation efficiency of complex ores using ionometry methods
https://doi.org/10.17073/2500-0632-2023-08-145
Abstract
Flotation beneficiation plays a leading role in the processing most ores. The efficiency of this process is ensured by the correct selection of operating modes, which involves choosing the most selective reagents and determining their optimal consumption. Despite the significance of this issue, the classic approach to determining beneficiation parameters involves testing followed by the processing of the results obtained and the determination of the reagent consumption. However, such studies do not reveal the essence of the physicochemical processes occurring within the pulp, and the results of testing one sample may not correspond to the optimum when the properties of the sample change.
The purpose of this work is to develop and implement a methodological approach to the study of ore flotation beneficiation using ionometry methods. The data obtained from ion-selective sensors significantly deepen our insight into the transformations occurring during the flotation process and allow for consideration of possible adverse factors that hinder effective process progression.
To achieve this goal, a comparative analysis of two approaches to flotation beneficiation testing was performed using complex sulfide ores as examples. In the first stage, a flotation beneficiation study was conducted through D-optimal factor testing, which included 20 individual tests to determine the optimal consumption of modifying reagents, yielding qualitative indicators. In the second stage, flotation tests were conducted using electrochemical monitoring with pH, Ag2S, Pt, and membrane electrodes. A universal flowchart for flotation studies with ion-selective sensors has been developed, facilitating the application of this approach to various ores. The implementation of the results from this comparative analysis has led to a 7.8% increase in beneficiation efficiency while reducing reagent consumption. Additionally, the insights gained into the electrochemical processes occurring allowed for assumptions about the adverse factors affecting flotation outcomes. In conclusion, a model for the potential application of this approach at existing enterprises was proposed, including the implementation of an “intelligent assistant” for flotation operators based on the developed electrochemical models.
Keywords
For citations:
Yakovleva T.A., Romashev A.O., Mashevsky G.N. Enhancing flotation beneficiation efficiency of complex ores using ionometry methods. Mining Science and Technology (Russia). 2024;9(2):146-157. https://doi.org/10.17073/2500-0632-2023-08-145
Enhancing flotation beneficiation efficiency of complex ores using ionometry methods
Introduction
The processing of mineral raw materials with low grade of commercially valuable components poses challenges for industrial enterprises aiming to modernize production. Currently, most enterprises address the problem of replenishing losses in concentrate production by increasing the volume of ore processed [1]. Additionally, efforts to increase or replenish the concentrate produced often result in the neglect of integrated subsoil usage, compromising the achievement of sustainable development goals in the mineral sector [2, 3].
Applying a more flexible approach through the use of modern automation technologies, as opposed to extensive method, enables the simultaneous resolution for both economic and, more importantly, environmental issues [4]. In the modernization of technologies at processing plants, researchers typically focus on optimizing the costliest stage, ore preparation [5]. While these efforts are crucial in reducing the final costs of the products obtained, the challenges of enhancing the comprehensiveness and depth of processing are predominantly addresses by intensifying the main stage: the flotation process [6–8].
In the contemporary practice of flotation beneficiation, the variability of the processed raw materials, especially due to ore heterogeneity, is one of the adverse factors affecting process performance [9]. This variability complicates the automatic control of the flotation process and the of dosing flotation reagents, thereby deteriorating both the qualitative and quantitative process indicators[10].
The selection of a reagent regime when studying ores for flotation dressability also poses challenges. Typically, this involves a step-by-step determination of the optimal consumption of each reagent type to establish the optimum conditions, or it involves applying various optimum plans. Although these methods are well-established, they require significant effort to conduct the testwork. For example, the commonly used Box-Behnken design necessitates 17 tests (in the simplest case, without repetitions). An alternative is the use of ionometry, measuring and recording electrode potentials. This approach, while established in electrochemical research, lacks a generally accepted methodology for its application in selecting reagent regimes and requires further refinement.
Therefore, the purpose of this work is to develop and test a methodological approach for studying flotation using ionometry.
To achieve this goal, we compared two approaches to designing tests for initial study of raw material dressability. The first, traditional approach uses a methodology to develop an optimal testwork plan, taking proposed flotation reagents’ consumption values as predictors (calculated in grams per tonne) to find the conditions providing the best quality indicators. The second approach utilizes ion-selective sensors (electrodes).
Theory
The flotation process, from the standpoint of automatic control and regulation, can be conditionally divided into three consecutive stages. The first stage, ore pulp preparation (ore grinding, adding water and modifying reagents, and the interaction of the ore with the reagents in the pulp), encapsulates the physicochemical essence of flotation. The second stage, the hydrodynamic one, includes the automatic stabilization of airflow rate and pulp level in a flotation machine, as well as other actuators controlling the process. The third stage involves adjusting the froth layer characteristics, where the secondary beneficiation of the processed raw materials occurs [11]. The first stage is of particular interest as it is the primary contributor to producing high-quality concentrate.
One approache to automatic process control involves studying the electrochemical properties of the flotation pulp. The importance of optimizing flotation based on ionometry was recognized as early as 1933–1934 when Wark and Cox first researched the relationship between the wetting contact angle and the pH value [5]. Their work also notes that pH is an important parameter in flotation processes. Over the years, pH remained the sole electrochemical parameter recognized in ore flotation dressability studies. However, analyzing additional parameters, such as electrode potentials, can uncover implicit dependencies and enhance overall process efficiency by creating digital twins of a particular processing stage or an entire plant flow sheet [12–14]. Recent publications have addressed this topic. For example, papers [15, 16] describe a “Digital Plant” project implementation: using the bulk flotation area of the Talnakh processing plant, a digital twin of an operator was created – a system that simulates the actions of an operator, stabilizes the flotation process, and performs control actions in real time [15]. Works [17, 18] emphasize the use of ion-selective electrodes for electrochemical pulp monitoring. The data obtained allowed for the investigation of electrochemical corrosion processes and the formation of coordination compounds of BtX with metals. After analyzing this data, the informative value of film membrane ion-selective EM electrode potentials was demonstrated. The investigation into the kinetics of potential shifts of a film membrane electrode within the decopperization and deironing circuit for rougher zinc concentrate at the Uchaly processing plant revealed substantial deviation of the electrode’s potential values into negative territory. These deviations did not match the actual concentrations of free xanthate ions in the pulp. The markedly negative potential recorded by film membrane electrode indicate the formation of complexes between xanthate ions and zinc cations. Additionally, patent [19] describes a pioneering practical shift from establishing control measures on “g/t” to applying mass ratios of pulp components that form sulfoxide complex, acting as depressants for Ni and Fe. Here, bisulfite ions were employed as sulfoxide depressants, with a maintained mass balance between iron ions, thiosulfate ions, and bisulfite ions in the pulp’s liquid phase. This industrial innovation highlights the potential for iron sulfite complex formation within the pulp, which can strongly adhere to the surface of nickel and pyrrhotite (sulfides) as hydrophilic anions, resulting in their depression.
While electrode use in enhancing flotation processes is not novel, the application of such knowledge to ore dressability studies is notably lacking, especially given the absence of a standardized methodological approach. The literature reviewed focuses on operational technologies, underlining the critical need to bridge this knowledge gap.
Research Materials and Techniques
Complex copper ores, comprised: 0.75% Cu, 23.48% Fe, 2.49% S, 64.61% SiO2, were chosen for this study. The mineralogical composition of the sample used in the research is presented in Table 1.
Table 1
Mineralogical composition of the sample
| Mineral designation | Mass fraction of mineral in samples, % | Mineral designation | Mass fraction of mineral in samples, % |
| Ore minerals | Nonmetallic minerals | ||
| Pyrite, marcasite | 2.27 | Calcite | 32–35 |
| Chalcopyrite | 2.55 | Garnet | 19–20 |
| Arsenopyrite | 1.58 | Chlorite | 15–16 |
| Magnetite | 1.05 | Microcline | 10–11 |
| Limonite | 0.40 | Pyroxene | 3–4 |
| Fahl ore | 0.21 | Kaolinite | 2–3 |
| Bornite | 0.11 | Quartz | 2–3 |
Preliminarily tests were carried out to determine the optinal grinding duration for the initial sample to achieve the liberation of mineral components, targeting a yield of 65% for the –0.071 mm grain size class. These tests were performed in a laboratory mill, MShL-7, with a ball load constituting 40% of the volume (9 kg) and a rotational speed set to 80% of the critical speed. The kinetic studies indicated that the required grinding time to reach the desired grain size was 12 min and 10 s.
Flotation beneficiation tests were conducted using a 3-L cell pneumatic-mechanical flotation machine from Vektis Minerals LLC. The material, immediately after grinding, was introduced to the cell where it interacted with flotation reagents according to a test plan.
The reagents employed included: CaO as a medium regulator, Na2S as a depressant (sulfidizer), and BtX (butyl potassium xanthate) as a collector. The tests protocol was as follows: the initial subsample with a grain size of –2.0 mm was milled to achieve 65% passing –0.071 mm, during which dry medium regulator (CaO) was also incorporated. Subsequently, the 1st rougher flotation was carried out with the addition of Na2S and BtX reagents, along with a constant T-80 frother dosage of 40 g/t. While the purpose of this research was not the selection and comparison of various reagents – although such an evaluation is extremely pertinent in the current economic climate – approach described below can be adapted for that purpose as well.
After the test, the beneficiation products were dried and analyzed using an EDX-8000P energy-dispersive X-ray fluorescent spectrometer, capable of detecting elements from C to U, manufactured by Shimadzu. Sample preparation for measurements involved obtaining a representative subsample weighing 5 ± 1 g followed by comminution.
During flotation beneficiation, the pulp’s electrochemical properties were monitored using a laboratory setup (Fig. 1), consisting of an array of electrodes connected to an EMF-16 Precision Electrochemistry Interface for real-time data acquisition redox and ion-selective electrodes (supporting up to 16 channels).
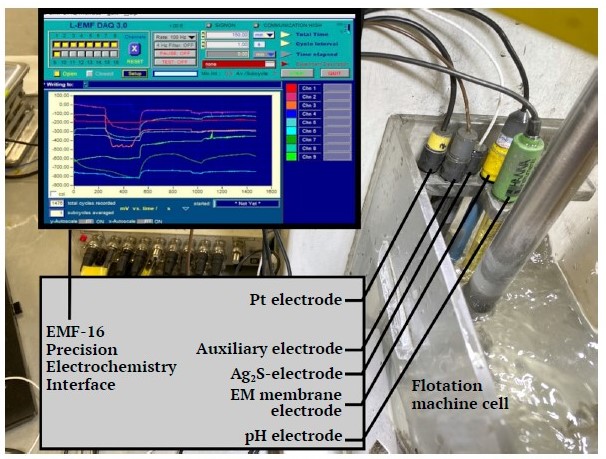
Fig. 1. Laboratory setup for monitoring electrochemical parameters in a flotation machine cell (complied by the authors)
The following ion-selective sensors (electrodes) were used to examine the feedstock’s electrochemical properties: a pH electrode for assessing hydrogen ions concentration in the pulp/water [20]; an Ag2S-electrode [21]; an EM – membrane electrode [22]; and an Pt electrode to determine the medium’s redox potential [23]. In line with the developed methodology’s requirements for electrochemical measurements, the ion-selective electrodes were calibrated to establish the electrochemical model necessary for diagnosing xanthate consumption and selecting the appropriate electrode set1 [24, 25].
The electrodes’ performance was gauged by calculating the slope of the electrode function, which for a singly charged component should be 59 ± 5, in accordance with the Nernst equation [26–28].
Calibration of pH electrodes followed a standard procedure, using buffer solutions and accounting for the temperature of the medium under examination.
1Romashev A. O., Yakovleva T. A., Gatiyatullin B. L. Computer program No. 2023680109. A program for selecting ion-selective sensors based on calibration data. Application submission: 2023-09-14, patent publication: 26.09.2023.
Results and Discussion
The initial phase of our flotation beneficiation research was conducted using a “classical” method, where we assembled a matrix for a three-factor D-optimal experiment [29]. This types of designs are widely used in various scientific disciplines for their superior capability in assessing the nonlinear impacts of factors [30–32]. The structure and design of the D-factor experiment are shown in Fig. 2 and Table 2, respectively. The consumptions of the aforementioned reagents were selected as predictors, and the consumption intervals were chosen based of an analysis of literature sources and operation experience from similar enterprises. As an optimization criterion, the Hankok–Luyken efficiency criterion for copper was used. This criterion serves as a technological optimization measure because it includes qualitative beneficiation indicators and generally reflects the degree of current beneficiation expertise relative to the maximum potential experience, the so-called case of “ideal” beneficiation. A total of 20 flotation tests were carried out (including repetitions in central points) within the prescribed consumption ranges of the experiment plan. From the products of each test, three representative samples were taken to analyze qualitative indicators, and the arithmetic mean of these three measurements was considered the final result. It should be noted that this work only addressed the rougher flotation stage, omitting additional flotation operations needed to produce standard products.
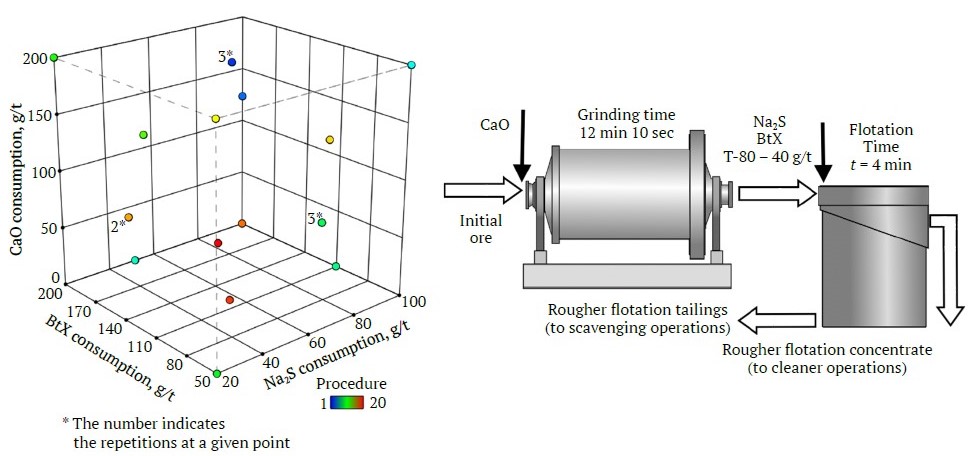
Fig. 2. Matrix of the D-factor experiment and flowchart for the series of tests (compiled by the authors)
Table 2
Testwork design and outcomes
| Factor | Reagent | UoM | Minimum | Maximum | Min. level | Max. level | Average | STDEV |
| A | Na2S | g/t | 20.00 | 100.00 | −1 ↔ 20.00 | +1 ↔ 100.00 | 58.25 | 30.06 |
| B | BtX | g/t | 50.00 | 200.00 | −1 ↔ 50.00 | +1 ↔ 200.00 | 117.81 | 58.27 |
| C | CaO | g/t | 0.00 | 200.00 | −1 ↔ 0.00 | +1 ↔ 200.00 | 104.85 | 75.38 |
Our statistical analysis for model adequacy showed an insubstantial effect of CaO consumption on flotation efficiency (Fig. 3), with no marked efficiency improvement upon increasing its usage. The local peaks arise from the influence of other factors.
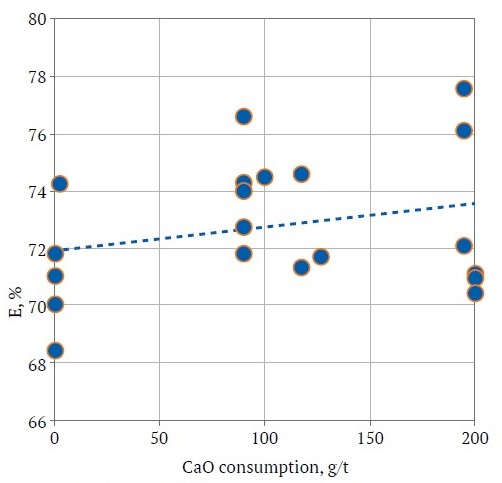
Fig. 3. Effect of CaO consumption on beneficiation efficiency (compiled by the authors)
Hence, it is judicious to assign a zero consumption level for this reagent for the samples under scrutiny. The analysis of the response surface of the efficiency of the beneficiation process, as a function of the consumption of Na2S and BtX, allows us to conclude that there is a local peak under the given test conditions (Fig. 4).
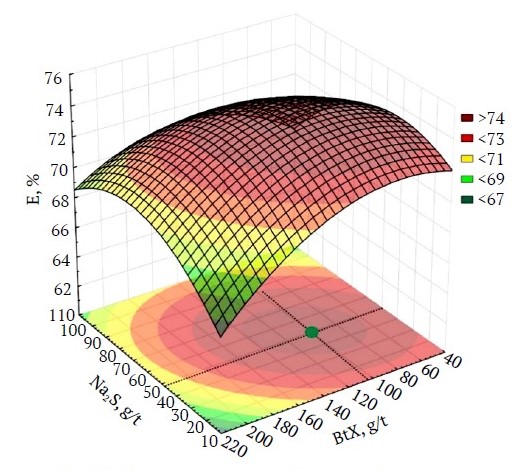
Fig. 4. Response surface of the beneficiation process efficiency as a function of Na2S and BtX consumption, based on results from the D-factor experiment (compiled by the authors)
To find the optimal consumption corresponding to the point of the local peak, the gradient search method was used, suitable for analyzing complex nonlinear dependencies. The solution found enabled the establishment of the consumptions for CaO, Na2S, and BtX at 0, 49.64, and 103.48 g/t, respectively.
According to the resulting model, the beneficiation efficiency at these parameters is 74.34%. To confirm the data obtained, an additional series of tests were carried out using the determined consumption values, and the averaged results (with each experiment was repeated 3 times) are presented in Table 3. The copper recovery to the concentrate of the 1st rougher flotation was 75.8%, with a copper grade of 16.20% and a beneficiation efficiency of 72.81%, which had a relative error of 2.05% compared to the model data.
Table 3
Results of open flotation tests at varying consumptions of selected reagents
| Product | Yield, % | Percentage, % | Recovery, % | ||||||
| Сu | Fe | S | SiO2 | Сu | Fe | S | SiO2 | ||
| 1st rougher flotation concentrate | 3.54 | 16.05 | 20.16 | 15.00 | 47.31 | 75.8 | 3.04 | 21.33 | 2.59 |
| Waste tailings | 96.46 | 0.18 | 23.60 | 2.03 | 65.25 | 24.2 | 96.96 | 78.67 | 97.41 |
| Ore | 100.00 | 0.75 | 23.48 | 2.49 | 64.61 | 100.00 | 100.00 | 100.00 | 100.00 |
The second stage of the research involved dressability tests with electrochemical parameter monitoring. A generalized flowchart of the research using ion-selective sensors is depicted in Fig. 5. This flowchart is divided into three stages. Stages 1 and 2 are conducted during the initial examination of a sample prior to flotation beneficiation. The information obtained at these stages first allows for the identification of the most sensitive electrode pairs (stage 1) and secondly determines the target values for the argentite electrode potential and the stabilization time. The selection technique is more fully described in [27] and [33].
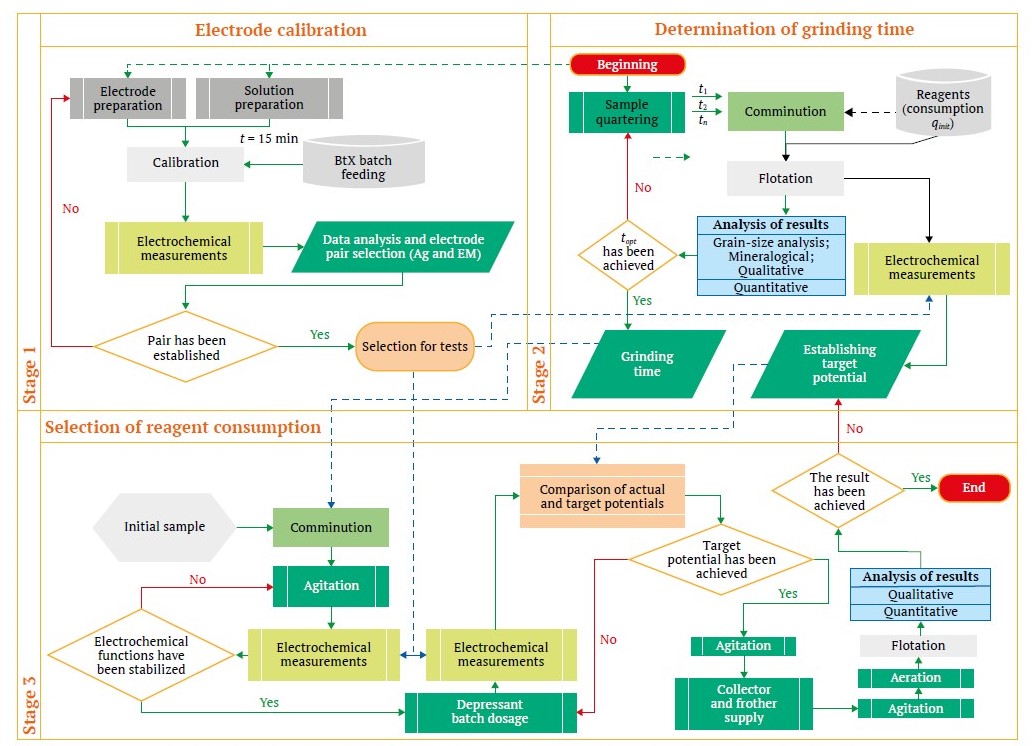
Fig. 5. Flowchart of flotation studies with ion-selective sensors (compiled by the authors)
Within the framework of this flowchart, tests to investigate the electrochemical properties of the initial pulp were conducted. The schematic of these tests is shown in Fig. 6.
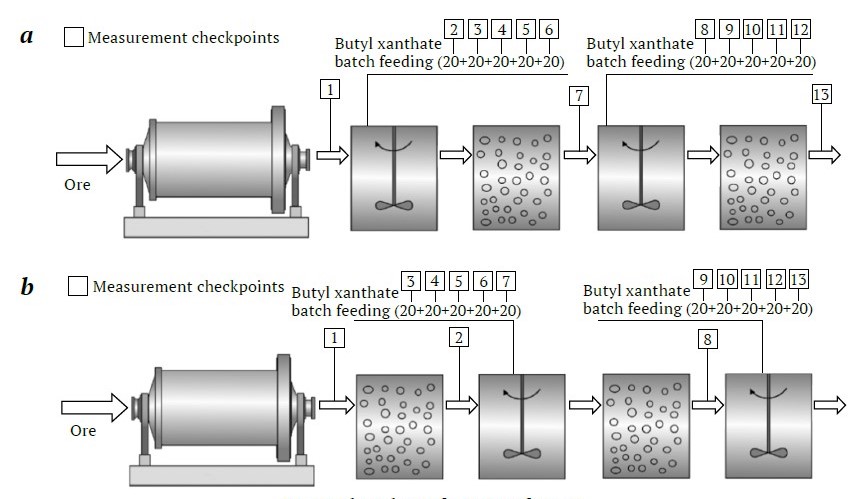
Fig. 6. Flow chart of a series of tests: a – without aeration; b – with preliminary aeration (compiled by the authors)
The method for investigating the electrochemical properties of ore pulp entails studying the kinetics of changes in the electrochemical parameters of the pulp, using a multisensor potentiometric system that monitors the potentials of electrodes installed directly in the pulp [27]. Then, an indicator (xanthate) is introduced into the resulting pulp both with and without preliminary aeration (Fig. 6, b and a, respectively).
Based on the obtained potentiograms of the absolute electrochemical potentials and diagnostic models, conclusions were drawn about the main components of the pulp that adversely or positively impact performance of the technological process. The results of the tests investigating the initial electrochemical parameters of the pulp are illustrated in Fig. 7.
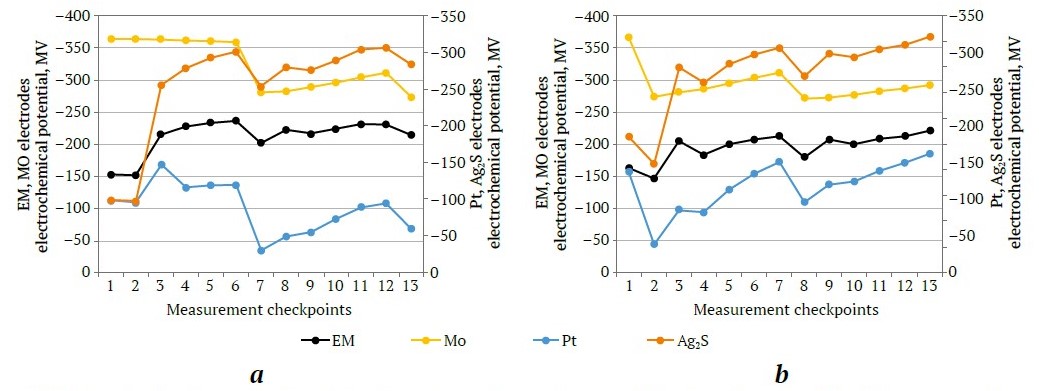
Fig 7. Results of tests investigating initial electrochemical parameters: a – without aeration; b – with preliminary aeration (compiled by the authors)
After mixing the initial sample pulp, the potentials of the Mo, Pt, and Ag2S electrodes shifted to the negative zone (Fig. 7, a, b). The potentiograms show a sharp increase in potential values during pulp aeration (point 3 in Fig. 7, a, b), which could be attributed to redox processes occurring [27]. The significantly negative potential of the Ag2S electrode upon mixing the initial pulp (after grinding) (point 1 in Fig. 7, a, b) suggests the presence of complexation processes, corroborated by changes in the kinetics of the film (membrane) ion-selective EM electrode potential. The Ag2S electrode readings, during aeration tests (point 3, Fig. 7, a, b), indicate that Cu2+ cations enter the pulp, binding the initial xanthate portions (i.e., xanthate binds to copper cations). Therefore, the incremental addition of Na2S could depress mineral surfaces and neutralize (mitigate) this adverse effect.
The results of this test are presented in Fig. 8 and Table 4. In Fig. 8, four zones of electrode function readings are discernible. Zone I shows a flattening of the electrode function. In Zone II, monitoring of the initial pulp parameters revealed the Ag2S electrode potential in negative zone at –320 to –330 mV, likely due to soluble elements in the ore generating Fe2+ ions in the pulp. During Zone III, the Na2S reagent was titrated for three minutes [33], a technique employed to sustain the Ag2S electrode potential at –450 mV [27]. In Zone IV, xanthate and air were introduced.
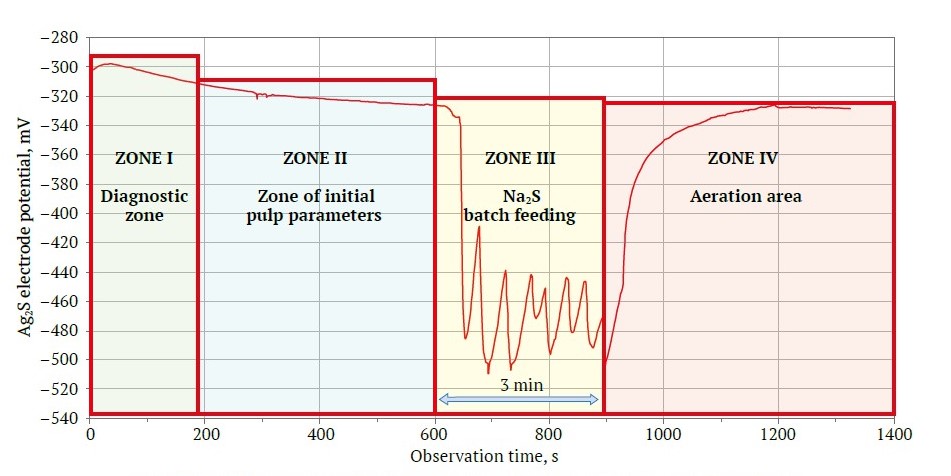
Fig. 8. Potentiogram displaying the kinetics of changes in Ag2S electrode potential (compiled by the authors)
Table 4
Results of open flotation tests at Ag2S potential maintained at –450 mV
| Product | Yield, % | Percentage, % | Recovery, % | ||||||
| Сu | Fe | S | SiO2 | Сu | Fe | S | SiO2 | ||
| 1st rougher flotation concentrate | 3.12 | 19.89 | 19.62 | 15.18 | 36.67 | 83.13 | 2.61 | 19.03 | 1.77 |
| Waste tailings | 96.88 | 0.13 | 23.60 | 2.08 | 65.51 | 16.87 | 97.39 | 80.97 | 98.23 |
| Ore | 100.00 | 0.75 | 23.48 | 2.49 | 64.61 | 100.00 | 100.00 | 100.00 | 100.00 |
The batched reagent consumptions were as follows: Na2S – 15 g/t and BtX – 100 g/t. The test results showed that the 1st rougher flotation concentrate grade increased from 16.05 to 19.89% Cu, while the Cu content in the tailings decreased from 0.26 to 0.18%. Consequently, Cu recovery rose by 7.37%. The beneficiation efficiency reached 80.61%, which was 7.8% higher than the target value obtained from the experimental design.
These results imply that the beneficiation flowchart for this raw material might effectively comprise only three operations: 1st and 2nd rougher flotations, as well as a scavenging flotation, since the 1st rougher flotation already achieves substantial recovery of sulfides into bulk concentrate.
Conclusion
The studies conducted have validated the effectiveness of ionometry methods for investigating ore dressability. By adjusting reagent feed and concurrently monitoring electrode potentials, we achieved a 7.8% increase in beneficiation efficiency within a single operation.
The research and the methodology introduced for examining the electrochemical properties of pulp enable rapid identification and management adverse factors affecting flotation, leading to enhance process quality indicators. The reducing in investigation time and the possibility of indirect real-time monitoring are also of notable importance. This method can serve as a blueprint for a production process, placing monitoring points throughout the production flowchart, and permits the real-time analysis of industrial beneficiation processes and, with provision for on-thefly adjustment reagents feeding points in the flotation process. A significant benefit of systems based on ion-selective sensors is their capability to deliver operational control data with minimal delay. The information and diagnostic models generated can help to minimize the influence of human error and reduce production costs through optimal reagent dosing. Looking ahead, the rapid advancement of artificial intelligence systems promises the development of self-learning systems employing neural network technology. These systems could function as an “interactive assistant”, offering real-time mode-adjustment recommendations with zero lag, leading to significant costs savings and significant economic benefits.
References
1. Litvinenko V. S., Petrov E. I., Vasilevskaya D. V. et al. Assessment of the role of the state in the management of mineral resources. Journal of Mining Institute. 2023;259:95–111. https://doi.org/10.31897/pmi.2022.100
2. Yurak V. V., Dushin A. V., Mochalova L. A. Vs sustainable development: scenarios for the future. Journal of Mining Institute. 2020;242:242–247. https://doi.org/10.31897/pmi.2020.2.242
3. Tsyglianu P. P., Romasheva N. V., Fadeeva M. L., Petrov I. V. Engineering projects in the Russian fuel and energy complex: actual problems, factors and recommendations for development. Ugol’. 2023;(3):45–51. (In Russ.) https://doi.org/10.18796/0041-5790-2023-3-45-51
4. Romasheva N. V., Babenko M. A., Nikolaichuk L. A. Sustainable development of the Russian Arctic region: environmental problems and ways to solve them. Mining Informational and Analytical Bulletin. 2022;(10–2):78–87. https://doi.org/10.25018/0236_1493_2022_102_0_78
5. Aleksandrova T. N., Afanasova A. V., Kuznetsov V. V., Aburova V. A. Selection of copper–nickel sulfide ore flotation parameters based on floatability ranking of flotation components. Mining Informational and Analytical Bulletin. 2022;(1):131–147. (In Russ.) https://doi.org/10.25018/0236_1493_2022_1_0_131
6. Vasilyeva M. A., Volchikhina A. A., Morozov M. D. Re-backfill technology and equipment. Mining Informational and Analytical Bulletin. 2021;(6):133–144. (In Russ.) https://doi.org/10.25018/0236_1493_2021_6_0_133
7. Zhou C., Zhao Y., Liu C. et al. Fluidization expansion of novel generation dense medium and flow regime transition in gas-solid separation fluidized bed. Fundamental Research. 2023. https://doi.org/10.1016/j.fmre.2023.02.008
8. Afanasova A. V., Aburova V. A., Prokhorova E. O., Lushina E. A. Investigation of the influence of depressors on flotation-active rock-forming minerals in sulphide gold-bearing ore flotation. Mining Informational and Analytical Bulletin. 2022;(6–2):161–174. (In Russ.) https://doi.org/10.25018/0236_1493_2022_62_0_161
9. Aleksandrova T. N. Key directions in processing carbonaceous rocks. Journal of Mining Institute. 2016;220:568–572. https://doi.org/10.18454/pmi.2016.4.568
10. Aleksandrova T. N., О’Connor C. Processing of platinum group metal ores in Russia and South Africa: current state and prospects. Journal of Mining Institute. 2020;244:462–473. https://doi.org/10.31897/pmi.2020.4.9
11. Boduen A. Ya., Petrov G. V., Kobylyansky A. A., Bulaev A. G. Sulfide leaching of high-grade arsenic copper concentrates. Obogashchenie Rud. 2022;(1):14–19. (In Russ.) https://doi.org/10.17580/or.2022.01.03
12. Nikolaeva N. V., Aleksandrova T. N., Chanturiya E. L., Afanasova A. V. Mineral and technological features of magnetite-hematite ores and their influence on the choice of processing technology. ACS Omega. 2021;6(13):9077–9085. https://doi.org/10.1021/acsomega.1c00129
13. Zhang D., Gao X. A digital twin dosing system for iron reverse flotation. Journal of Manufacturing Systems. 2022;63:238–249. https://doi.org/10.1016/j.jmsy.2022.03.006
14. Ohenoja M., Koistinen A., Hultgren M. et al. Continuous adaptation of a digital twin model for a pilot flotation plant. Minerals Engineering. 2023;198:108081. https://doi.org/10.1016/j.mineng.2023.108081
15. Bendaouia A., Abdelwahed E. H., Qassimi S. et al. Digital Transformation of the Flotation Monitoring Towards an Online Analyzer. In: Smart Applications and Data Analysis. SADASC 2022. Communications in Computer and Information Science. Springer, Cham. 2022;1677. https://doi.org/10.1007/978-3-031-20490-6_26
16. Abrarov A. D., Datsiev M. S., Chikildin D. E., Fedotov D. N. Optimization of bulk flotation process at Talnakh Concentrator based on machine learning algorithms. Tsvetnye Metally. 2022;(2):87–93. (In Russ.) https://doi.org/10.17580/tsm.2022.02.11
17. Aleksandrova T. N., Ushakov E. K., Orlova A. V. Method of complex copper–zinc ore typification using neural network models. Mining Informational and Analytical Bulletin. 2020;(5):140–147. (In Russ.) https://doi.org/10.25018/0236-1493-2020-5-0-140-147
18. Aleksandrova T., Nikolaeva N., Kuznetsov V. Thermodynamic and experimental substantiation of the possibility of formation and extraction of organometallic compounds as indicators of deep naphthogenesis. Energies. 2023;16(9):3862. https://doi.org/10.3390/en16093862
19. Mashevskyi G. N., Petrov A. V., Lyyra M. et. al. Development of new series of Outotec products for electrochemical control of flotation process. Tsvetnye Metally. 2010;(2):93–95. (In Russ.)
20. Göktepe F. Effect of pH on pulp potential and sulphide mineral flotation. Turkish Journal of Engineering and Environmental Sciences. 2002;26(4):309–318.
21. Horwood C., Stadermann M. Evaluation of a Ag/Ag 2 S reference electrode with long-term stability for electrochemistry in ionic liquids. Electrochemistry Communications. 2018;88:105–108. https://doi.org/10.1016/j.elecom.2018.02.005
22. Tatarnikov A. V., Sokolskaya I., Shneerson Ya. M. et al. Treatment of platinum flotation products. Platinum Metals Review. 2004;48(3):125–132. https://doi.org/10.1595/003214004X483125132
23. Liao L. W., Li M. F., Kang J. et al. Electrode reaction induced pH change at the Pt electrode/electrolyte interface and its impact on electrode processes. Journal of Electroanalytical Chemistry. 2013;688:207–215. https://doi.org/10.1016/j.jelechem.2012.08.031
24. Balatovic M. Handbook of flotation reagents: chemistry, theory and practice. Flotation of Sulfide Ores. Elsevier; 2007. 445 p. https://doi.org/10.1016/b978-0-444-53029-5.x5009-6
25. Woodcock J. T., Jones M. H. Chemical environment in Australian lead-zinc flotation plant pulps: II, Collector residualsm metals in solution, and other parameters. In: Proceedings of the Australasian Institute of Mining and Metallurgy.1970;235:61–76.
26. Titov D. V. Applying geophysical methods to assessing the technological properties of ores of sulfide polymetallic deposits. Izvestiya Tomskogo Politekhnicheskogo Universiteta. 2006;309(4):40–47. (In Russ.)
27. Yakovleva T. A., Romashev A. O., Mashevsky G. N. Digital technologies for optimizing the dosing of flotation reagents during flotation of non-ferrous metal ores. Mining Informational and Analytical Bulletin. 2022;(6–2):175–188. (In Russ.) https://doi.org/10.25018/0236_1493_2022_62_0_175
28. Vidal-Iglesias F. J., Solla-Gullón J., Rodes A. et al. Understanding the Nernst equation and other electrochemical concepts: an easy experimental approach for students. Journal of Chemical Education. 2012;89(7):936–939. https://doi.org/10.1021/ed2007179
29. Tan S. Y., Chia V. Y. Y., Hölttä-Otto K., Anariba F. Teaching the Nernst equation and faradaic current through the use of a designette: an opportunity to strengthen key electrochemical concepts and clarify misconceptions. Journal of Chemical Education. 2020;97(8):2238–2243. https://doi.org/10.1021/acs.jchemed.9b00932
30. Napier-Munn T. J. Statistical methods for mineral engineers – How to design experiments and analyse data. Queensland, Australia: Julius Kruttschnitt Mineral Research Centre; 2014. 627 p.
31. Goos P., Jones B., Syafitri U. I-optimal design of mixture experiments. Journal of the American Statistical Association. 2016;111(514):899–911. https://doi.org/10.1080/01621459.2015.1136632
32. Mancenido M. V., Pan R., Montgomery D. C., Anderson-Cook C. M. Comparing D-optimal designs with common mixture experimental designs for logistic regression. Chemometrics and Intelligent Laboratory Systems. 2019;187:11–18. https://doi.org/10.1016/j.chemolab.2019.02.003
33. Mashevskiy G. N., Ushakov E. K., Yakovleva T. A. Digital technology for optimizing the sodium sulphide dosage during copper ore flotation. Obogashchenie Rud. 2021;(3);18–23. (In Russ.) https://doi.org/10.17580/or.2021.03.04
34. Aleksandrova T., Nikolaeva N., Kuznetsov V. Thermodynamic and experimental substantiation of the possibility of formation and extraction of organometallic compounds as indicators of deep naphthogenesis. Energies. 2023;16(9);3862. https://doi.org/10.3390/en16093862
35. Aleksandrova T. N., Prokhorova E. O. Modification of properties of rock-forming minerals during flotation. Mining Informational and Analytical Bulletin. 2023;(12):123–138. (In Russ.) https://doi.org/10.25018/0236_1493_2023_12_0_123
About the Authors
T. A. YakovlevaRussian Federation
Tatyana A. Yakovleva – PhD-Student of the Department of Mineral Processing
Saint Petersburg
A. O. Romashev
Russian Federation
Artyem O. Romashev – Cand. Sci. (Eng.), Associate Professor of the Department of Mineral Processing, Deputy Head of the Department of Mineral Processing
Scopus ID 56330093400
Saint Petersburg
G. N. Mashevsky
Russian Federation
Gennady N. Mashevsky – Dr. Sci. (Eng.), Technology Advisor
Scopus ID 56290154600
Saint Petersburg
Review
For citations:
Yakovleva T.A., Romashev A.O., Mashevsky G.N. Enhancing flotation beneficiation efficiency of complex ores using ionometry methods. Mining Science and Technology (Russia). 2024;9(2):146-157. https://doi.org/10.17073/2500-0632-2023-08-145
JATS XML




































