Scroll to:
Preparation of adsorbents for the extraction of heavy metals from mining wastewater
https://doi.org/10.17073/2500-0632-2024-02-224
Abstract
Mining and metallurgical operations are inextricably connected with the consumption of large volumes of water and, consequently, the generation of liquid waste. The priority is to solve the problems of treatment and rational reclaiming of process waters with high content of valuable components. This will make it possible to obtain a significant environmental and economic effect, i.e. to bring profit directly to enterprises, save material resources and reduce the environmental impact in mining regions. Processing of copper-zinc ores is accompanied by the formation of metal-bearing wastewater with a wide range of associated metals and nonmetals with low concentrations of each individual component and pH fluctuations within wide ranges. These factors make it difficult to select a rational treatment technology, so enterprises have to pay for excessive metal-bearing discharges into the environment. Heavy metals are toxic, do not undergo decomposition, can be accumulated by aquatic plants and reach a human body through the food chain. Centralized accumulation of accidental discharges, surface and drainage water with subsequent treatment for use in recycled water supply can solve a number of environmental problems in the field of water resources protection. Adsorption of heavy metals by zeolites produced from inexpensive clay minerals due to the simplicity of the process, possibility of zeolite regeneration, high efficiency in Cu2+, Zn2+ and Fe2+ ion exchange with release of non-toxic Na+ cations into the environment is a good alternative to chemical precipitation. The purpose of this study is to optimize the conditions for producing zeolites from kaolin and bentonite with the assessment of the possibility of their use for the treatment of wastewater generated during mining and processing of ores from sulfide copper-polymetallic deposits. The technology of alkaline fusion of bentonite or kaolin with sodium hydroxide was used as a basis for zeolite synthesis from crude mining products. The novelty of the technological approach in obtaining zeolites from natural aluminosilicates in comparison with the published data is that the adjustment of the chemical composition of alkaline alloy for the synthesis of zeolites with a certain crystal structure was carried out using Al2O3–NaAlО2 waste suspension. The alkaline alloy was dissolved in water, filtered, and subjected to hydrothermal crystallization. The phase composition of the zeolite adsorbents was studied. Through studying the recovery of heavy metals from model solutions, the mass composition and conditions of alkaline fusion processes as well as the hydrothermal crystallization mode were optimized. The achieved metal recovery of 95% from the model solutions with initial concentration (mg/L): 150 Cu2+, 180 Zn2+ and 125 Fe2+ allowed to draw the conclusion that zeolites based on bentonite and kaolin can be used in the treatment of metal-bearing wastewater.
Keywords
For citations:
Mirzaeva E.N., Isaeva N.F., Yalgashev E.Ya., Turdiyeva D.P., Boymonov R.M. Preparation of adsorbents for the extraction of heavy metals from mining wastewater. Mining Science and Technology (Russia). 2025;10(1):45-55. https://doi.org/10.17073/2500-0632-2024-02-224
Preparation of adsorbents for the extraction of heavy metals from mining wastewater
Introduction
The development of mining industry is accompanied by the formation of specific technogenic systems, including mine, shaft, pit and under-dump waters, as well as mining-generated dust pollution [1]. Compounds of copper, zinc, lead, iron, and other heavy metals have a negative impact on the hydrosphere, as well as on the health of population, especially children [2]. Enterprises in ore processing apply various processes to maximize the recovery of nonferrous metals from technogenic waters and their return to the production cycle [3]. However, monitoring of natural waters in the areas of mining and processing and nonferrous metallurgy activities impact indicates a clear exceeding of metal content sanitary norms in wastewater [4]. Discharge of ineffectively treated water by Almalyk mining and metallurgical combine (AMMC) caused exceeding the MPC of zinc, lead, copper, iron, manganese, and molybdenum in the water use section of the Akhangaran River [5]. In order to reduce the anthropogenic impact on the hydrosphere, it is advisable to remove harmful impurities in treatment devices at industrial enterprises using water resources.
In the practice of water treatment from moderately concentrated contaminants natural zeolites are increasingly used [6-8], the main advantage of which is cheapness. Despite the high selectivity of clinoptilolite towards heavy metals (Pb2+, Cd2+, Cu2+, Co2+, Cr3+, Zn2+, Ni2+, Hg+, Fe3+), the adsorption capacity of natural zeolites is several times less than that of synthetic zeolites [7, 9]. Therefore, researchers are increasingly interested in the synthesis of zeolites from clay minerals [10-12], waste coal rock [13–15], crushed stone [16] and other cheap wastes [17, 18]. V. V. Somerset et al. revealed the effect of reducing the concentration of Ni, Zn, Cd, and Hg after treatment of acid mine drainage water with zeolites (phojasite) [19]. The conversion of source components into zeolites with framework structure is carried out taking into account phase composition, chemical activity and thermal stability of the source mineral raw materials, as well as the intended field of application. Expensive single-phase zeolites such as NaA, NaX, or NaY are produced by two-stage crystallization from pure reagents at a strict ratio of Na2SiO3, NaOH, and NaAlO2 [20, 21], since a one-stage hydrothermal crystallization does not allow obtaining zeolites of a certain structure without impurities of extraneous phases. Hydrothermal treatment of kaolin calcined at 550–650 ºС (with insignificant content of quartz and other refractory minerals) with alkaline solution is preferable for synthesis of NaA zeolite due to favorable Si/Al ratio close to two. More thermally stable bentonite [10], kaolin as part of waste coal rock [13] are calcined at temperatures of about 800 ºС, and additional sources of aluminum are resorted to at the hydrothermal stage. A promising way of processing low-grade mining products into adsorbents for wastewater treatment from heavy metals is fusion of silicon-aluminum-containing raw materials together with NaOH and subsequent hydrothermal crystallization of zeolites from soluble alkaline alloy products [14, 15, 22].
The purpose of this study is the synthesis of zeolite adsorbents from low-grade natural raw materials with high content of crystalline quartz. The goals of this study are as follows: 1) characterization of phase and elemental composition and thermal stability of Uzbek clay minerals; 2) determination of the influence of alkaline fusion conditions on the phase composition of the hydrothermal crystallization products; 3) evaluation of the efficiency of extraction of copper, zinc, iron, and lead (being common pollutants of mine and waste waters) from model aqueous solutions. The key task was to develop a low-cost adsorbent for use in mining and metallurgical industry.
Research Materials and Methods
The natural clay minerals used in this work are Navbakhor bentonite (NB) and Angren variegated kaolin (VK); plus sodium aluminate suspension (SAS) was used. Sodium aluminate suspension is a residue produced when alumina wastes are processed into adsorbents of halide containing compounds. Its phase composition, %, is as follows: NaAlO2 – 30–33, NaOH – 1.5–2.5; Na2CO3 – 0.3–0.5, water – the rest. The zeolites derived from bentonite and kaolin are hereinafter designated as NBS and VKS. The codes of prepared samples, conditions of their synthesis and phase compositions are given in Table 1. In addition, the following commercial ingredients were used: 99% sodium hydroxide, hydrochloric acid (36.5% HCl), and 99% polyethylene polyamine (PEPA). The bentonite had the following elemental composition, wt.%: O, 51.03; Si, 26.95; Al, 7.25; Mg, 1.42; K, 1.65; Fe, 7.21; Na, 1.24; Ca, 2.32; Ti, 0.32; S, 0.20 and P, 0.35. The kaolin contained, wt.%: O, 53.38; Si, 25.49; Al, 10.59; Mg, 0.1; K, 0.81; Ba, 0.22; Fe, 1.91; Pb, 0.05; Na, 0.12; Ca, 0.22. The sodium aluminate suspension with a density of 1.23 g/cm3 after drying at 350 ºC contained, wt.%: O, 41.57; Al, 30.37; Na, 25.82; C, 2.08; Cl, 0.14.
Table 1
Effect of parameters of synthesis by alkaline fusion of 100 g of silicon-containing raw materials with NaOH and SAS on the crystallized products phase composition
| Sample code | Ingredients of the mixture for alkaline fusion, g | Hydrothermal treatment | Molar ratio | Detected phases,% | |||||||||
| Zeolites | Impurities | ||||||||||||
| NaOH | SAS | H2O, g | L/S ratio | Zeolite, 0.2 g | SiO2/Al2O3 | Na2O/ SiO2 | Н2О/ Na2O | NaA | NaX | NaP | Hydrosodalite | SiO2 | |
1-NBS | 75.82 | 131.3 | 2175 | 14.7 : 1 | NaA | 1.5 | 1.5 | 130 | 13 | – | – | 43 | 11 |
2-NBS | 49.8 | 89.9 | 1448 | 7.83 : 1 | NaA | 2.0 | 1.0 | 83.0 | 56 | – | – | 12 | 14 |
3-NBS | 88.58 | 89.9 | 2199 | 10.2 : 1 | – | 2.0 | 1.5 | 84.0 | 8 | – | – | 75 | 8 |
4-NBS | 98.85 | 64.97 | 2180 | 10.6 : 1 | NaX | 2.5 | 1.5 | 83.3 | 4 | 74 | – | 10 | 6 |
5-NBS | 33.95 | 40.79 | 2513 | 17.7 : 1 | NaX | 3.3 | 0.6 | 240 | 4 | 63 | 6 | – | 9 |
6-NBS | 1.24 | – | 240 | 2.66 : 1 | NaA | 7.17 | 0.016 | 860 | 4 | – | – | 7 | 88 |
7-NBS | 55.05 | 100.8 | 1609 | 8.36 : 1 | NaA | 2.0 | 1.0 | 83.0 | 69 | – | – | 8 | – |
8-NBS | 54.57 | 73.1 | 1609 | 8.87 : 1 | NaX | 2.5 | 1.0 | 83.0 | 2 | 79 | – | 3 | – |
9-NBS | 49.8 | 89.9 | 1448 | 7.83 : 1 | NaA | 2.0 | 1.0 | 83.0 | 36 | – | – | 55 | – |
10-NBS | 75.82 | 131.3 | 2175 | 9.40 : 1 | – | 1.5 | 1.5 | 130 | – | – | – | 82 | – |
11-NBS | 98.85 | 64.97 | 2180 | 10.6 : 1 | – | 2.5 | 1.5 | 83.3 | – | – | – | 76 | 10 |
1-VKS | 52.65 | 67.13 | 1370 | 8.64 : 1 | NaA | 2.0 | 1.0 | 83.0 | 64 | – | – | 8 | – |
2-VKS | 89.3 | 67.13 | 2057 | 11.0 : 1 | NaA | 2.0 | 1.5 | 83.1 | 8 | – | – | 75 | – |
3-VKS | 59.92 | 43.53 | 1370 | 9.2 : 1 | NaX | 2.5 | 1.0 | 83.0 | 6 | 78 | – | 4 | – |
4-VKS | 37.62 | 20.67 | 824 | 7.0 : 1 | – | 3.3 | 0.6 | 83.2 | 10 | 24 | 38 | - | – |
5-VKS | 59.92 | 43.53 | 1370 | 9.17 : 1 | NaX | 2.5 | 1.0 | 83.0 | – | 75 | – | 25 | – |
6-VKS | 60.05 | 12.62 | 1194 | 8.64 : 1 | NaA | 2.0 | 1.0 | 83.0 | 83 | – | – | 3 | – |
7-VKS | 49.48 | 46.91 | 1194 | 7.87 : 1 | – | 1.5 | 1.0 | 83.0 | 25 | – | – | 64 | – |
8-VKS | 89.3 | 67.13 | 1370 | 8.64 : 1 | – | 2.0 | 1.0 | 83.0 | 6 | – | – | 90 | – |
The conversion of clay raw materials into soluble sodium aluminates and aluminosilicates was carried out by alkaline fusion with NaOH [11], with adjusting the ratio of SiO2/Al2O3 and Na2O/SiO2 in the mixture by addition of sodium aluminate suspension. The SiO2/Al2O3 molar ratio varied from 1.5 to 7.17, and Na2O/SiO2 molar ratio varied 0.016 to 1.5. The bentonite and kaolin for the synthesis of 7-NBS and 6-VKS were preliminarily subjected to partial leaching of Ca, Mg, K and Fe impurities by heat treatment in 2M HCl solution followed by washing and drying at 120 ºС. The ingredients mixture was wet milled in a ball mill for one hour, transferred to porcelain cups, dried at 80–120 ºС, and then calcined with an exposure time of 3 h at 810 ºС. The alkaline fusion products were pulverized, transferred to a retort with a stirrer, and distilled water was added in the ratio of Liquid : Solid (L : S) from 2.9 : 1 to 6.6 : 1.
After stirring for 30 min, the liquid was filtered from the solid residue into a polypropylene retort [21], and inoculum crystals were added at the rate of 0.2 g of NaA or NaX fresh zeolite powder per 100 g of the reaction mass. In the synthesis of 8-NBS and 5-VKS, 60 g of РЕРА were added to the clear filtrate, and the reaction mixtures for 9-11-NBS and 8-VKS samples were exposed to microwave radiation for 0.5 h before being placed into an autoclave (see Table 1). Each of the obtained clear solutions in a polypropylene retort was placed into an autoclave and kept at 35 ºС for 15–25 h to reach equilibrium in the reaction mixture. The temperature was then raised to 80 ºС and incubated for 4 h. The optimum temperature of the induction period and crystal growth stage was selected for each alkaline alloy solution depending on the results of phase analysis of samples taken every hour. The crystallized products were separated on a Nutch-filter, washed with water to neutral pH value of the liquid phase, dried, and calcined at 300 ºС.
For the dynamic mode studies, 8-NBS and 6-VKS powders were molded by extrusion into pellets of 1.0 mm in diameter and 1.0–1.3 mm long. The molding mixture was prepared by mixing zeolite powder with impurity-purified bentonite and carboxymethylcellulose solution. The pellets dried and calcined at 300 ºС were treated with Na2CO3 solution before adsorption. Under static conditions powdery adsorbents were used to evaluate the extent of heavy metal removal from a test solution. Conical retorts containing 100 ml each of the test solution and a presice weight suspension of the corresponding zeolite (0.05–1.0 g) were set into a shaker at 25 ºС. The shaking time varied from 10 to 600 min. The dynamic tests were carried out in a column with a diameter of 1.25 cm at the height of the zeolite particles layer of 120 cm and liquid flow rate from 6 to 18 cm 3/min. The degree of removal of harmful impurities from the water was evaluated by photocolorimetric method by the change in the concentration of the corresponding metals in contact with a zeolite sample. Zinc was determined as a complex compound with dithizone (analytical wavelength λ = 535 nm), and copper, with lead diethyldithiocarbamate (λ = 430 nm). We measured the optical density of the solutions of the complex compounds: zinc with dithizone (analytical wavelength λ = 535 nm), copper with lead diethyldithiocarbamate (λ = 430 nm), and iron with sulfosalicylic acid (λ = 430 nm). Lead concentration was determined by chromate method at λ = 540 nm using diphenylcarbazide.
Adsorption capacity was determined according to the following formula:
A = (Cini – Cfin)V∙100/m,
where A – adsorption capacity; Cini – Initial concentration of a substance, g/l; Cfin – concentration of the substance under study in the solution in equilibrium conditions, g/l; m - mass of adsorbent, g; V - volume of model solution, l [11]. The elemental composition of the studied samples was determined using a scanning electron microscope (SEM) EVOMA 10 (Zeiss). X-ray phase analysis (XRD) diffractograms of the source substances and the synthesized zeolites were obtained on an Emmyrean diffractometer using CuKα radiation, and the thermograms, on a HESON HS-TGA-103 derivatograph with a heating rate of 10 ºС/min.
Experimental results
It followed from the diffractograms, Fig. 1, that the source materials taken were a mixture of different minerals and differed significantly in molar ratio: SiO2/Al2O3 = 4.64 (kaolin) and 7.17 (bentonite), one of the most important characteristics for the synthesis of zeolites.
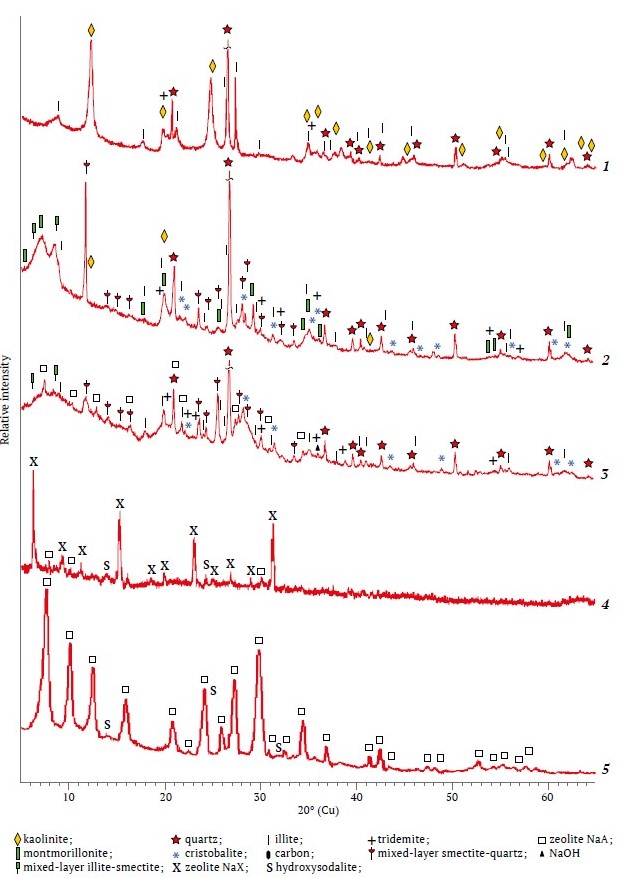
Fig. 1. Diffractograms: 1 –VK; 2 – NB; 3 – 4-NBS (alkaline fusion at 800 ºС – 5 h); 4 – 8-NBS; 5 – 6-VKS
In the diffractogram of kaolin, slightly broadened reflections with d = 0.714; 0.446; 0.357; 0.2568; 0.2504; 0.2387; 0.2343; 0.221; 0.1989; 0.1790; 0.1666; 0.1490; 0.1375; 0.701; 0.129; 0.1236 nm were attributed to the manifestation of the rock-forming mineral kaolinite. Kaolinite is a layered aluminosilicate with the following chemical formula: Al2Si2O5(ОН)4 [6], where the SiO2/Al2O3 molar ratio is equal to 2, same to NaA zeolite. The relative intensity of the basal reflections of kaolinite was less than that of quartz; in addition, reflections attributed to illite, a hydromicaceous potassium-bearing mineral (K<1Al2[Al,Si)4O10].(OH)2.n H2O (d = 0.998; 0.498; 0.421; 0.333; 0.320; 0.298; 0.256; 0.243; 0.239; 0.224; 0.218; 0.212; 0.198; 0.166; 0.164; 0.150 nm) were observed. The process of dehydroxylation of kaolinite into amorphous metakaolin occurred in the range of 490-610 oC, while illite and quartz did not undergo phase transformation up to 800 ºС (Fig. 2, thermogram 1).
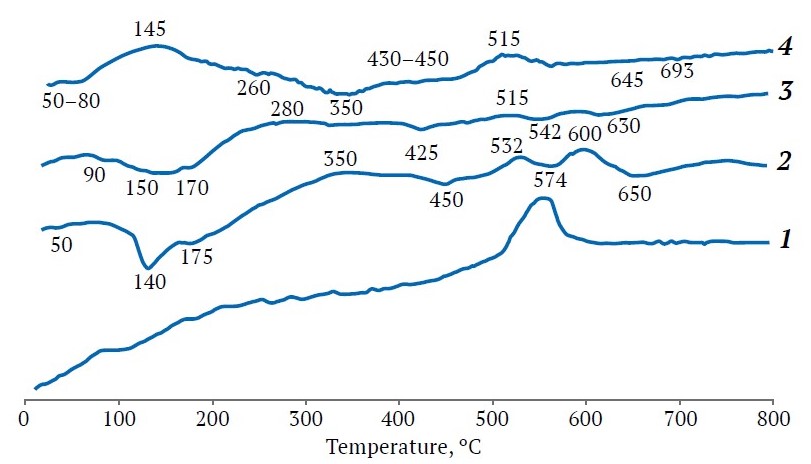
Fig. 2. Thermograms of components used in the synthesis of zeolite adsorbents: 1 – variegated colored kaolin (VK); 2 – Navbakhor bentonite (NB); 3 – alkaline fusion for sample 4-NBS; 4 – alkaline fusion for sample 2-VKS
The rock-forming mineral of bentonite was montmorillonite from the smectite group, identified by the main reflections with d = 0.568; 0.704; 0.809; 0.4472; 0.3422; 0, 3053; 0.2598; 0.1711; 0.1672, and 0.1504 nm along with the reflections of quartz and mixed-layer phases: smectite-quartz and smectite-illite (see Fig. 1, diffractogram 2). The indicated reflections partially coincided with those from illite, kaolinite, and α-cristobalite that is characteristic of fine clay deposits [10]. After calcination of bentonite at 600–650 ºС kaolinite and montmorillonite were not identified radiographically, but the background in the region of angles 2θ = 18–32o from the amorphous products of their dehydroxylation increased, the reflections from illite were preserved, but the reflection from crystalline polymorphs of SiO2 prevailed. The phases of tridymite, α-cristobalite, and micaceous minerals identified in the bentonite and kaolin were preserved after heat treatment at 850 ºС and therefore required thermochemical activation with alkaline reagents [11]. In the alloys of bentonite or kaolin with NaOH at 800 ºС, the intensity of quartz lines was 50% of the initial one, but sharply decreased with increasing the fusion temperature to 810 ºС. The thermogram of natural bentonite in combination with the data of X-ray phase analysis after its calcination at 550 ºС confirmed that the change in the shape and some shift of the reflections of montmorillonite were due to the transformation of most of the swelling phase of montmorillonite into illite, which is not prone to swelling. Molecular water was removed at 50, 140 and 175 ºС with a mass loss of 9.2%, and dehydroxylation of structural OH-groups with a mass loss of 1.8% occurred at 450, 574 and 650 ºС (see Fig. 2, thermogram 2).
The sodium aluminate suspension after water removal was a mixture of X-ray amorphous sodium aluminates in terms of NaAlO2 and crystalline phases, %: gibbsite, 28.7; NaOH, 10.9; Na2CO3, 1.98, and NaCl, 0.024. After the calcination at 810 ºС, broad reflections from γ-NaAlO2 (d = 0.425; 0.294; 0.259; 0, 215; 0.1994; 0.1970; 0.1881; 0.1747 nm) and NaAl11O17 (d = 1.128; 0.569; 0.280; 0.2518; 0.2424; 0.2380 nm) appeared on the diffractogram instead of the halo from amorphous phases. The transformation of gibbsite into γ-Al2O3 was indicated by reflections with d = 0.198 and 0.139 nm. Crystalline Na2СO3 was identified by low-intensity narrow reflections with d = 0.2963; 0.26; 0.254; 0.236; 0.218 nm. The codes of zeolites obtained under optimal conditions, the amount of ingredients in the mixtures for alkaline fusion and hydrothermal treatment of their leaching products, as well as the results of X-ray phase analysis of the synthesized zeolites are presented in Table 1.
Findings Discussion
The optimal conditions of thermochemical decomposition of initial silicon sources were selected by comparing the diffractograms of the alkaline alloys obtained at temperatures of 800, 810, and 830 ºС and intended for synthesis of samples 1-NBS, 4-NBS, 2-VKS, and 6-VKS with the results of the analysis of the solid residues from aqueous leaching. It was shown that almost complete conversion of montmorillonite, kaolinite, and SiO2 polymorphs into sodium aluminosilicates can be achieved by the calcination of the alkaline mixtures at a temperature of at least 810 ºС for 3 h. Narrow reflections from nepheline NaAlSiО4 with d = 0.4192; 0.3834; 0.3262; 0.3011; 0.2884 nm and very broad reflections with d = 0.53; 0.432; 0.282; 2.55; 2.30 nm from 2NaAlO2∙3H2O were observed at the alkaline alloy diffractograms for 1-NBS, 10-NBS, and 7-VKS samples with silicon deficiency (SiO2/Al2O3 = 1.5). At SiO2/Al2O3 ≥ 2 и Na2O/SiO2 = 1, background enhancement in the region of angles 2θ = 5–35o from amorphous aluminosilicates and SiO2 was observed in the diffractograms, and the crystals of high-temperature aluminosilicate Na4Al2Si2О9 (d = 0.4213; 0.2584; 0.1822, and 0.1487 nm) were formed instead of nepheline. The traces of the high-temperature form of NaAlSiО4 (d = 0.423 and 0.2592 nm), weak reflections of α-cristobalite (d = 0.4013; 0.3132; 0.2852;0.2481; 0.193; 0.187 nm), which did not react with NaOH or Na2CO3, and a hydromica that did not contain constitutional water (d = 0.436; 0.302; 0.260; 0.225; 0.202 nm) manifested themselves. In the diffractogram of the 4-NBS alloy with some surplus of sodium compounds, in addition to the Na4Al2Si2О9 crystalline phase, reflections with d = 0.5302; 0.3563; 0.3041; 0.2574; 0.2403; 0.1885, and 0.1754 nm from Na2SiО3 were also detectable. In the alkaline alloys, as a rule, the Na4Al2Si2О9 phase predominated, whose reflections intensity changed in the series: 8-VKS ≈ 6-VKS < 1-VKS < 7-NBS < 2-NBS. Both forms of soluble sodium aluminosilicates were also present in the diffractograms of the other alkaline alloys.
Decreasing grinding time or duration of heat treatment of the alkaline alloys was accompanied by the appearance of reflections of quartz at 2θ = 20.8º; 26.14º; 36.6º; 50.21º; 59.82º and those of γ-Al2O3 around 2θ = 55.2º in the diffractograms. It was shown in separate experiments that fusion of bentonite or kaolin with sodium hydroxide alone at 810 ºС was less effective than combining NaOH with a suspension of sodium aluminate at the ratio of alkaline components NaOH : Na2CO3 = 5 : 1 and equivalent amount of Na2O. The products of alkaline fusion at temperatures above 800 ºС, namely, amorphous silicates, weakly crystallized sodium aluminates, and crystalline NaAlSiО4, Na4Al2Si2О9 и Na2SiО3, were well soluble in water and were thermodynamically unstable due to a large number of breaks and free ends of bonds in the places of their breakage [23]. At an optimum temperature of 80 ºС, pH = 12–13, and 4 h autoclave exposure, the dissolved products of alkaline fusion crystallized predominantly into zeolites of NaХ (SiO2/Al2O3≈2.5), NaА (SiO2/Al2O3 ≈ 2.0) or NaР (SiO2/Al2O3≈3.33) type depending on the elemental composition (see Table 1). The characteristic diffraction peaks with d = 1.230; 0.870; 0.710; 0.550; 0.37; 0.328; 0.298, and 0.262 nm were assigned to NaA zeolite, and the peaks with d = 1.447; 0.885; 0.754; 0.573; 0.380, and 0.288 nm [24], to NaX zeolite. If the alkaline fusion process was carried out at a temperature of 800 ºС, amorphous SiO2, its crystalline polymorphs, and unreacted micaceous minerals strongly polluted the end product (see Fig. 1, diffractogram 3). When the mass same to that at the 4-NBS diffractogram was fused at 810 ºС, the SiO2 peak area (2θ = 26.6º) was 18 times less than that of the NaX zeolite phase (2θ = 6.02º) [25].
It was found by varying the time of hydrothermal treatment of the dissolved alkaline alloys filtrate for samples 2-NBS, 4-NBS, 1-VKS, 4-VKS, and 5-VKS that the introduction of inoculum crystals NaA or NaX reduced the crystallization time from 7 to 3–4 h with a total yield of zeolite phases of 56–78%. Reducing the time of the hydrothermal treatment to 1–2 h sharply reduced the yield of the target products, and in diffractograms, the intensity of basic reflections of zeolite phases at 2θ = 6.1–7.18º amounted to 8–15% of the quartz peak at 2θ = 26.6º. The crystallization of the alkaline alloy solution at 810 ºС for more than 4 h increased the yield of NaA and/or NaX zeolites by 7–8%, but led to an increase in the content of extraneous phases such as hydroxysodalite (peaks with d = 0.633; 0.365; 0.258; 0.239, and 0.211 nm [24]. The increase of crystallization time during the synthesis of sample 6-VKS from 3.5 h (see Table 1, Fig. 3) to 5 h was accompanied by an increase in the yield of NaA zeolite from 83 to 84%, and that of hydroxysodalite from 3 to 11% that complied with the findings of studies [14, 26]. The increased silicon content (SiO2/Al2O3 = 2.5–3.3) and applying NaX crystal inoculation at the hydrothermal treatment of alkaline bentonite alloy solutions with sodium aluminate suspension led to prevailing formation of the NaX zeolite phase with d = 0.1447; 0.885; 0.7.54; 0.573; 0.481; 0.442; 0.394; 0.381 nm (see Table 1).
Na+ cations played an important role in zeolitization by stabilizing the basic bonds of zeolite frameworks under hydrothermal conditions [27]. Not only the degree of destruction of the initial crystalline substances during fusion to form water-soluble substances, but also the alkalinity of the resulting solution for crystallization depended on the amount of sodium compounds during fusion. The slightly alkaline medium (pH = 8–8.5) during the synthesis of 6-NBS sample at Na2O/SiO2 ratio of 0.016 without addition of sodium aluminate suspension led to the formation of amorphous silica with a minimum of crystalline phases. pH ≥ 14 during the hydrothermal stage of synthesis of 1-NBS, 3-NBS, 10-NBS, 11-NBS, 2-VKS, and 7-VKS caused a clear predominance of hydroxysodalite phase. Table 1 shows that from 64 to 90% of hydroxysodalite were formed without NaA or NaX inoculum crystals during hydrothermal treatment of alkaline alloy solutions with SiO2/Al2O3 ratio in the range of 1.5–2.5. The reflections with d = 0.6293; 0.3649; 0.2814; 0.2573; 0.209; 0.1812; 0.17398; 0.1572; 0.15263; 0.1483, and 0.14425 nm in the diffractograms were assigned to the hydroxysodalite phase. According to literature sources hydroxysodalite is a hydrophilic zeolite with variable composition like Na6Al6Si6O24.8H2O [22], Na8[AlSiO4]6(OH)2.2H2O, and others [16, 28]. As was noted in the reviews [23, 26], the skeleton of NaA zeolite – Na12Si12Al12O48.27H2O, formed as a result of hydrothermal crystallization from nepheline solutions with the calculated ratio of SiO2/Al2O3 = 2, is subject to cracking at high alkalinity, which caused the formation of hydrosodalite. Hydroxysodalite crystals are known to have a cubic shape with an ordered aluminosilicate framework, in which sodalite cells enclose small-sized pores [29], which can be useful in the extraction of metal ions from aqueous solutions [28]. In our experiments, 10-NBS and 8-VKS adsorbents consisting predominantly of hydroxysodalite were obtained without introduction of inoculum crystals but with microwave treatment. The introduction of NaA zeolite inoculum crystals into the microwave-treated reaction mixture clearly reduced not only the amount of useless quartz, but also prevented the formation of NaA zeolite crystals that was somewhat different from the conclusions drawn in [27], devoted to the conversion of fly ash into A-type zeolites with the use of microwave treatment.
Study of adsorption properties
Multiphase nature of the synthesized adsorbents, including two types of metastable zeolite structures A and X, along with the stable phases of hydroxysodalite and NaP zeolite caused the specificity of their adsorption properties. The samples with the predominance of hydrosodalite or NaP zeolite were characterized by higher density and low specific surface area, about 33—76 m2/g, in comparison with metastable NaA and NaX zeolites with similar ratio of SiO2/Al2O3 with a specific surface area of up to 300 m2/g. The large volume of cavities characteristic of hydroxysodalite allowed adsorbents such as 10-NBS, 11-NBS, and 8-VKS adsorb some amount of metal ions from water, but extremely slowly. The tests under static conditions (see Fig. 3) were conducted with solutions of concentrations typical for polymetallic mine waters [2], including the Almalyk ore field, which includes copper-porphyry and copper sulfide-polymetallic deposits with sulfides prevailing [30]. However, monitoring of natural waters in the zone of influence of mining-and-processing and nonferrous metallurgy enterprises indicates an obvious exceeding of sanitary norms in wastewater. At a concentration of less than 0.003M and pH about 5 (Fig. 3, curves 1–4) metals were present in solutions practically in the form of divalent cations. The adsorption capacity for 10 h of the experiment did not exceed (g/100 g) 0.38Cu2+ for 8-VKS and 0.27 Pb2+ in the case of 10-NBS, whereas for the same period the capacity of 100 g of 6-VKS zeolite with A-type structure containing about 3% hydroxysodalite reached, g: 29.2 Cu2+; 28.4 Zn2+; 18.3 Fe2+ and 3.1 Pb2+. Due to the increase of pH up to 5.1 in contact with 0.05 g of zeolite as a result of ion exchange Cu2+ → 2Na+, the competition between metal cations and excess of protons decreased, and immobilization of hardly soluble hydroxocomplexes on the adsorbent surface occurred, so the copper compounds adsorption efficiency reached 97%. When the zeolite weight was increased to 0.06 g, the degree of removal of these metals from dilute solutions was not less than 98.5% that agrees with literature data [18, 19] for fly ash-based zeolites. The maximum iron and zinc compounds adsorption capacity was recorded for 8-NBS zeolite, where the predominant phase was NaX zeolite, g/100 g: 20.8 Fe2+ and 28.7 Zn2+. Hybrid sample 4-VKS, which included predominantly NaP zeolite structure and some NaX and NaA, absorbed lead better than 10-NBS – 1.2 Pb2+ (g/100 g), but was significantly inferior to samples 6-VKS and 8-NBS. Increased content of hydroxysodalite and SiO2 phases (crystalline or amorphous) deteriorated the ability of adsorbents to extract metal cations. At adsorption from 1M solutions (potential liquid ore), where molecular forms of heavy metals prevailed, removal of toxic elements occurred mainly by precipitation mechanism with formation of Сu(ОН)2 precipitate, and only at increasing pH up to 6.5 due to high dose of zeolite the degree of the removal reached 80%.
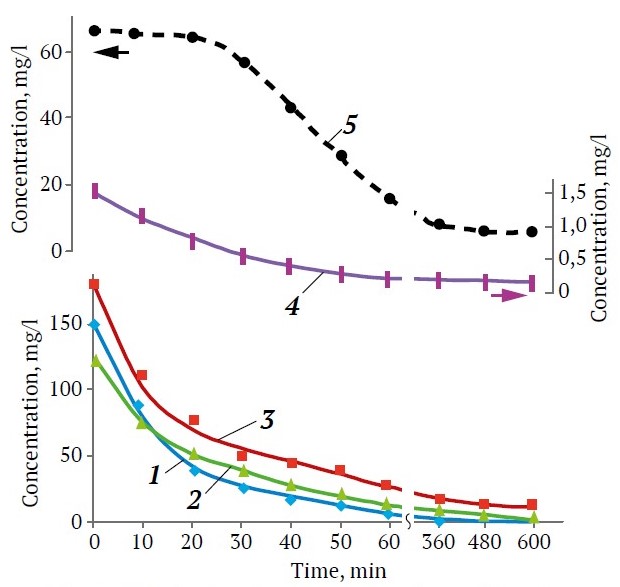
Fig. 3. Effect of contact time of zeolites with water containing heavy metal ions on the change of solution concentration in the systems: 1 – Сu2+/ 6-VKS, 2 – Fe2+/8-NBS, 3 – Zn2+/4-VKS, 4 – Pb2+/10-NBS, 5 – Сu2+/4-VKS
The tests of optimal samples in dynamic mode showed that when passing model solutions through a layer of zeolites 8-NBS and 6-VKS with a height of 1.2 m, the efficiency of purification from Cu2+, Zn2+ and Fe2+ cations was not less than 95%, and that for Pb2+, about 82%.
The concentration of copper cations as a result of adsorption on zeolites 8-NBS and 6-VKS decreased, mg/L: from 150 to 1.2–0.5 Cu2+, for zinc, from 180 to 8.9–7.5 Zn2+, for iron, from 125 to 0.8–0.4 Fe2+, for lead, from 1.5 to 0.3–0.27 Pb2+. The results of adsorption on the zeolites synthesized from local mineral raw materials by alkaline fusion with waste Al2O3–NaAlО2 suspension were confirmed in the process of treatment of actual under-dump water of Kulchulak deposit. The degree of purification of the water with concentrations, mg/l: Cu – 138, Zn – 169, Fe – 83, Pb – 1.8 in the presence of sulfur and silver compounds and pH 4.9 was at least 80%. After the concentrations of Сu2+, Zn2+ and Fe2+ at the outlet of the adsorption column exceeded about 10 mg/L, the possibility of regeneration of zeolites, saturated with heavy metal cations, by passing Na2CO3 solution was proved in principle. Zeolites 8-NBS and 6-VKS can be used for the treatment of the process water of Almalyk mining and metallurgical combine.
Conclusion
Preliminary alkaline fusion of crude clay minerals with waste sodium aluminate suspension provided effective generation of active silicon and aluminum particles, allowed to increase the yield of zeolite structure products and sharply (up to the complete absence) reduce the amount of useless impurities of quartz and mullite. The tendency to form hydroxysodalite was enhanced at excessive increase of alkalinity and duration of the hydrothermal stage.
Under water treatment process parameters comparable to commercial adsorption technologies, it is possible to reduce the concentration of copper, zinc, and iron to practically the level of MPCs in drinking water. The zeolites synthesized from crude mineral raw materials with high quartz content can be used in process water treatment to reduce water consumption by mining and metallurgical enterprises from external sources.
References
1. Rastanina N.K., Kolobanov K.A. Impact of technogenic dust pollution from the closed mining enterprise in the Amur Region on the ecosphere and human health. Mining Science and Technology (Russia). 2021;6(1):16–22. https://doi.org/10.17073/2500-0632-2021-1-16-22
2. Zvereva V. P., Frolov K. R., Lysenko A. I. Formation of mine drainage in the Far Eastern region and its impact on the ecosphere and public health. Mining Science and Technology (Russia). 2022;7(3):203–215. https://doi.org/10.17073/2500-0632-2022-3-203-215
3. Shadrunova I. V., Orekhova N. N. Technological and environmental and economic aspects processes for advanced recycling of water originating from mining operations, with recovery of heavy metals. Mining Informational and Analytical Bulletin. 2015;(S1):177–191. (In Russ.)
4. Kataeva S.E., Shulyak E. V., Bryl V. I., Chaban N. G. On the issue of heavy metal content in the aquatic environment of Slavutych city. Moscow; 2000. Pp. 751–752. (In Russ.)
5. Fayzieva M. F. On the issue of sanitary protection of reservoirs in Uzbekistan. Vestnik Nauki i Obrazovaniya. 2016;(4):70–72. (In Russ.)
6. Collins F., Rozhkovskayaa A., Outramb J. G., Millarb G. J. A critical review of waste resources, synthesis, and applications for Zeolite LTA. Microporous and Mesoporous Materials. 2020;291:109667. https://doi.org/10.1016/j.micromeso.2019.109667
7. Tasić Ž. Z., Bogdanović G. D., Antonijević M. M. Application of natural zeolite in wastewater treatment – A review. Journal of Mining and Metallurgy. 2019;55A(1):67–79 https://doi.org/10.5937/JMMA1901067T
8. Belova T. P., Ratchina T. I., Gavrilenko Yu. S. Sorption of copper, nickel and cobalt by natural zeolite from aqueous solutions. Mining Informational and Analytical Bulletin. 2014;12:76–80. (In Russ.)
9. Milicevic S., Povrenovic D., Milosevic V., Martinovic S. Predicting the copper adsorption capacity on different zeolites. Journal of Mining and Metallurgy. 2017;53A(1):57–63.
10. Srilai S., Tanwongwal W., Onpecth K. et al. Synthesis of Zeolite X from bentonite via hydrothermal method. Materials Science Forum. 2020;990:144–148. https://doi.org/10.4028/www.scientific.net/msf.990.144
11. Ma H., Yao Q., Fu Y. et al. Synthesis of zeolite of type A from bentonite by alkali fusion activation using Na2CO3. Industrial & Engineering Chemistry Research. 2009;49(2):454–458 https://doi.org/10.1021/ie901205y
12. Burоnоv F., Fayzullayev N. Synthesis and aррlicatiоn of high silicоn zeоlites frоm natural sources. In: AIP Conference Proceedings. The 1st International Conference on Problems and Perspectives of Modern Science: ICPPMS-2021. 10–11 June 2021, Tashkent, Uzbekistan. 2022;2432:050004. https://doi.org/10.1063/5.0089557
13. Jin Y., Li L., Liu Z. et al. Synthesis and characterization of low cost zeolite NaA from coalgangue by hydrothermal method. Advanced Powder Technology. 2021;32:791–801 https://doi.org/10.1016/j.apt.2021.01.024
14. Kong D., Jiang R. Preparation of NaA Zeolite from High Iron and Quartz Contents Coal Gangue by Acid Leaching-Alkali Melting Activation and Hydrothermal Synthesis. Crystals. 2021;11(10):1198. https://doi.org/10.3390/cryst11101198
15. Ma H., Zhu H., Wu C. et al. Study on compressive strength and durability of alkali-activated coal gangue-slag concrete and its mechanism. Powder Technology. 2020;368:112–124. https://doi.org/10.1016/j.powtec.2020.04.054
16. Kuroki S., Hashishin T., Morikawa T. et al. Selective synthesis of zeolites A and X from two industrial wastes: Crushed stone powder and aluminum ash. Journal of Environmental Management. 2019;231:749–756. https://doi.org/10.1016/j.jenvman.2018.10.082
17. Koukouzas N., Vasilatos C., Itskosa G. et al. Removal of heavy metals from wastewater using CFB-coal fly ash zeolitic materials. Journal of Hazardous Materials. 2010;173(1–3):581–588 https://doi.org/10.1016/j.jhazmat.2009.08.126
18. Hamadi A., Nabih K. Synthesis of zeolites materials using fly ash and oil shale ash and their applications in removing heavy metals from aqueous solutions. Hindawi Journal of Chemistry. 2018;2018(1):6207910. https://doi.org/10.1155/2018/6207910
19. Somerset V., Petrik L., Iwuoha E. Alkaline hydrothermal conversion of fly ash filtrates into zeolites 2: Utilization in wastewater treatment. Journal of Environmental Science and Health, Part A. 2005;40(8):1627–1636. https://doi.org/10.1081/ESE-200060675
20. Bessa R., Costa L., Oliveira C. et al. Kaolin-based magnetic zeolites A and P as water softeners. Microporous and Mesoporous Materials. 2017;245:64–72. http://dx.doi.org/10.1016/j.micromeso.2017.03.004
21. Tayraukham P., Jantarit N., Osakoo N., Wittayakun J. Synthesis of pure phase NaP2 zeolite from the gel of NaY by conventional and microwave-assisted hydrothermal methods. Crystals. 2020;10(10):951. http://dx.doi.org/10.3390/cryst10100951
22. Wajima T., Munakata K., Ikegami Y. Conversion of waste sandstone cake into crystalline zeolite X using alkali fusion. Materials Transactions. 2010;51(5):849–854. https://doi.org/10.2320/matertrans.MH200905
23. Lee Y.-R., Soe J. T., Zhang S. et al. Synthesis of nanoporous materials via recycling coal fly ash and other solid wastes: A mini review. Chemical Engineering Journal. 2017:317;821–843. http://dx.doi.org/10.1016/j.cej.2017.02.124
24. Kunecki P., Panek R, Wdowin M. et al. Influence of the fly ash fraction after grinding process on the hydrothermal synthesis efficiency of Na-A, Na-P1, Na-X and sodalite zeolite types. International Journal of Coal Science & Technology. 2021;8(2):291–311 https://doi.org/10.1007/s40789-020-00332-1
25. Hu T., Gao W., Liu X. et al. Synthesis of zeolites Na-A and Na-X from tablet compressed and calcinated coal fly ash. Royal Society Open Science. 2017;4:170921. http://dx.doi.org/10.1098/rsos.170921
26. Yang L., Qian X., Yuan P. et al. Green synthesis of zeolite 4A using fly ash fused with synergism of NaOH and Na2CO3. Journal of Cleaner Production. 2019;212:250–260. https://doi.org/10.1016/j.jclepro.2018.11.259
27. Mallapur V. P., Oubagaranadin J. U. K. A brief review on the synthesis of zeolites from hazardous wastes. Transactions of the Indian Ceramic Society. 2017;76(1):1–13. https://doi.org/10.1080/0371750X.2016.1231086
28. Golbad S., Khoshnoud P., Abu-Zahra N. Hydrothermal synthesis of hydroxy sodalite from fly ash for the removal of lead ions from water. International Journal of Environmental Science and Technology. 2017;14(1):135–42. https://doi.org/10.1007/s13762-016-1133-x
29. Shabani J. M., Omotola B., Oyekola O., Petrik L. Synthesis of hydroxy sodalite from coal fly ash for biodiesel production from waste-derived maggot oil. Catalysts. 2019;9(12):1052. https://doi.org/10.3390/catal9121052
30. Vasilevsky B. B., Yezhkov Yu. B., Rakhimov R. R. et al. Problems of large-volume gold and copper deposits in Uzbekistan. Tashkent; 2012. 116 p. (In Russ.)
About the Authors
E. N. MirzaevaUzbekistan
Elena I. Mirzaeva – PhD (Eng.), Associate Professor of the Department of Metallurgy
Almalyk
N. F. Isaeva
Uzbekistan
Nurkhon F. Isaeva – PhD (Eng.), Doctoral Student
Tashkent
E. Ya. Yalgashev
Uzbekistan
Elmurod Ya. Yalgashev – Doctoral Student, Senior Researcher
Tashkent
D. P. Turdiyeva
Uzbekistan
Dilnoza P. Turdiyeva – Doctoral Student, Junior Researcher
Tashkent
R. M. Boymonov
Uzbekistan
Rufatjon M. Boymonov – Senior Lecturer of the Department of Machinery and Equipment of Oil and Gas Industry and Pipeline Transport Systems
Tashkent
Review
For citations:
Mirzaeva E.N., Isaeva N.F., Yalgashev E.Ya., Turdiyeva D.P., Boymonov R.M. Preparation of adsorbents for the extraction of heavy metals from mining wastewater. Mining Science and Technology (Russia). 2025;10(1):45-55. https://doi.org/10.17073/2500-0632-2024-02-224
JATS XML




































